Abstract
Introduction
This study outlined cemiplimab intravenous (IV) dosing strategy to move from body weight (BW)-based 3 mg/kg every-2-week (Q2W) dosing in first-in-human study (study 1423; NCT02383212) to fixed 350 mg every-3-week (Q3W) dosing, utilizing population pharmacokinetics (PopPK) modeling and simulations, and supported by a limited dataset from a phase 2 study (study 1540; NCT02760498).
Methods
Cemiplimab concentration data from a total of 505 patients were pooled from study 1423 in advanced malignancies and study 1540 in advanced cutaneous squamous cell carcinoma (CSCC). All patients received weight-based cemiplimab dose (1, 3, 10 mg/kg Q2W or 3 mg/kg Q3W) except 4% who received 200 mg Q2W. A linear two-compartment PopPK model incorporating covariates that improved goodness-of-fit statistics was developed to compare cemiplimab exposure at 350 mg Q3W versus 3 mg/kg Q2W. Upon availability, observed cemiplimab concentration at 350 mg Q3W in study 1540 was then compared with the simulated values.
Results
Post hoc estimates of cemiplimab exposure and variability (505 patients; weight range 30.9–156 kg; median 76.1 kg) at steady state were found to be similar at 350 mg Q3W and 3 mg/kg Q2W. Effect of BW on cemiplimab exposure was described by exposure versus BW plots and at extreme BW. Overlay of individual observed cemiplimab concentrations in 51 patients with metastatic CSCC on simulated concentration–time profiles in 2000 patients at 350 mg Q3W confirmed cemiplimab exposure similarity and demonstrated the robustness of dose optimization based on PopPK modeling and simulations.
Conclusions
Cemiplimab 350 mg Q3W is being further investigated in multiple indications.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Compared with body weight-based dosing, fixed dosing offers the advantages of increased convenience, easy preparation, reduced risk of dosing errors, minimized waste, and improved compliance and patient adherence. |
This study demonstrated selection of a fixed 350 mg every-3-week (Q3W) dose of cemiplimab using population pharmacokinetics (PopPK) modeling and simulations. |
The selection was further supported by observed data of cemiplimab at 350 mg Q3W, which became available after PopPK modeling and simulations. |
These results supported approval of cemiplimab 350 mg Q3W by the US FDA (cemiplimab-rwlc) and by the European Commission for treatment of patients with metastatic cutaneous squamous cell carcinoma (CSCC) or locally advanced CSCC who are not candidates for curative surgery or curative radiation. |
Digital Features
This article is published with digital features, including a summary slide and graphical abstract, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13643165.
Introduction
Cemiplimab is a high-affinity, human, hinge-stabilized immunoglobulin (Ig)G4 monoclonal antibody (mAb) to the programmed cell death (PD)-1 receptor that potently blocks the interactions of PD-1 with PD-ligand 1 (PD-L1) and PD-ligand 2 (PD-L2) [1]. Cemiplimab (cemiplimab-rwlc in the US) is approved for the treatment of patients with metastatic or locally advanced cutaneous squamous cell carcinoma (mCSCC, laCSCC; collectively referred to as advanced CSCC) who are not candidates for curative surgery or curative radiation [2, 3]. It is also approved in the US for patients with locally advanced and metastatic basal cell carcinoma (BCC), post hedgehog inhibitors (HHIs) or for whom HHIs are not appropriate [2]. Cemiplimab-rwlc is also approved for the first-line treatment of patients with advanced non-small cell lung cancer (NSCLC) whose tumors have high PD-L1 expression (tumor proportion score ≥50%) and no epidermal growth factor receptor, anaplastic lymphoma kinase or ROS1 aberrations, for patients with metastatic or locally advanced tumors that are not candidates for surgical resection or definitive chemoradiation [2].
In first-in-human (FIH; study 1423; NCT02383212) and phase 2 (study 1540; NCT02760498) studies, cemiplimab demonstrated antitumor activity, durable responses, and a safety profile similar to those described for other anti–PD-1 therapies in patients with advanced malignancies, including advanced CSCC [4]. In these studies, the pharmacokinetics (PK) of cemiplimab was linear and dose proportional over a dose range of 1–10 mg/kg administered intravenously (IV) every 2 weeks (Q2W) [2]. As a human monoclonal antibody directed against PD-1, a cell membrane target, cemiplimab is expected to exhibit a saturable, target-mediated elimination pathway leading to non-linear PK at low concentrations [5]. The observed linear PK is, therefore, suggestive of saturation of the underlying target-mediated pathways at the concentrations evaluated. A weight-based 3 mg/kg Q2W IV dose regimen that ensured maximum therapeutic effect at target saturation across the whole patient population was initially recommended as the phase 2 dose (RP2D).
Dosing of drugs with narrow safety margins, as is often the case with chemotoxic agents, is often based on body weight or body mass index to reduce inter-patient variability in drug exposure and to improve clinical efficacy while limiting adverse events [6]. mAbs are target specific, and as such, many have a considerable therapeutic window due to high target specificity. Although body weight impacts the PK of mAbs, the contribution of body weight to PK variability is limited [6]. Therefore, further PK evaluation has encouraged adoption of fixed dosing with mAb therapies, which offers the advantages of increased convenience, easy preparation, reduced chance of dosing errors, minimized waste, and improved compliance [6,7,8]. In recent years, fixed dosing of mAbs has become more common in multiple therapeutic areas, including oncology [9,10,11]. In addition, anti–PD-1 therapies, including nivolumab and pembrolizumab, showed flat exposure–response relationships for clinical efficacy and safety endpoints, providing evidence for the limited effect of variability in drug exposure on clinical efficacy and/or safety at the tested dose levels [12, 13]. Clinical efficacy of cemiplimab was observed at the lowest tested dose level of 1 mg/kg Q2W, while no dose-limiting toxicities were observed at the highest tested dose level of 10 mg/kg Q2W, suggesting a wide therapeutic window. In the FIH study 1423, cemiplimab exposure and variability in exposure were similar among patients with advanced malignancies dosed with cemiplimab 200 mg Q2W (n = 20) and a larger group dosed with cemiplimab 3 mg/kg Q2W, warranting cemiplimab fixed dose options to be considered. Evaluation of a fixed dose regimen for cemiplimab also involved extended dosing with reduced dosing frequency to further improve convenience and patient adherence.
Population PK (PopPK) modeling, which provides an integrated assessment of PK in patient populations, usually across multiple studies, is an important tool to guide dose selection and to support critical drug development decisions [14,15,16].
This study aims to select an every-3-week (Q3W) fixed dose regimen of cemiplimab with similar exposure as the initial 3 mg/kg Q2W RP2D. Dose selection was based on simulations of cemiplimab exposure by a PopPK model with linear and dose proportional PK using cemiplimab concentration in serum data collected in studies 1423 and 1540, where the majority of the patients were dosed at 3 mg/kg Q2W. A fixed 350 mg Q3W dose regimen that generated similar cemiplimab exposure at steady state (AUC6wk,ss, area under the cemiplimab concentration–time curve over 6 weeks at steady state; Cmax,ss, maximum concentration at steady state; Cmin,ss, minimum concentration at steady state) as 3 mg/kg Q2W was retained. Observed cemiplimab exposure at 350 mg Q3W, which became available in patients with mCSCC (group 3 of study 1540), was compared to the simulated cemiplimab exposure at 350 mg Q3W and to that observed at 3 mg/kg Q2W.
Methods
Data
Blood samples to measure cemiplimab concentrations in serum were collected in study 1423 at the first cycle prior to and at the end of infusion, after 1, 4, 8, 24, 48, and 72 h, and after 8, 15, 29, and 43 days; on days 1, 15, 29, and 43 prior to and/or at the end of infusion for cycles 2–6. Blood samples in study 1540 were collected at the first cycle on days 1, 15, 29, and 43 prior to and/or at the end of infusion. Sampling was also carried out for cycles 2–7, 9, and 11 on day 1 prior to and/or at the end of infusion. The last cemiplimab blood samples were collected at the end of study visit and/or at follow-up visits. A validated enzyme-linked immunosorbent assay with a lower limit of quantification of 0.078 mg/L was used to measure functional cemiplimab concentrations in serum.
The cemiplimab concentration data used for PopPK modeling were collected from 396 patients with advanced malignancies, including 88 patients with lung cancer, 47 with breast cancer, 35 with skin cancer, 28 with liver cancer, 24 with cervical cancer, 23 with colorectal cancer, 21 with head and neck cancer, 19 with central nervous system cancer, 13 with pancreatic cancer, 11 with ovarian cancer, 9 with soft tissue sarcoma, 7 with cancer of the uterus, 6 with prostate cancer, 5 with bladder cancer, 4 with adrenal cancer, 4 with esophageal cancer, 3 with basal cell carcinoma, 3 with stomach cancer, 2 with salivary gland cancer, 2 with kidney cancer, 2 with nasopharyngeal cancer, 1 with paranasal sinus cancer, 1 with rectal cancer, 1 with gall bladder cancer, 1 with thyroid cancer, 1 with anal cancer, 1 with advanced solid tumor, and 34 with other cancers in study 1423, and from 109 patients with advanced CSCC in study 1540. In these studies, cemiplimab was administered as a 30-min IV infusion. Patients received either weight-based dosing of cemiplimab (1, 3, 10 mg/kg Q2W or 3 mg/kg Q3W; 96% of patients) or fixed dosing (200 mg Q2W; 4% of patients). In 505 patients with advanced malignancies included in this analysis, median age was 65 (range 27–96) years, 296 (58.6%) patients were male, 456 (90.3%) patients were Caucasian, median body weight was 76.1 kg (range 30.9–156 kg; 2.5th percentile 47.7 kg; 97.5th percentile 117 kg), and median body mass index was 26.5 (range 14.8–56.3) kg/m2. Duration of treatment was up to 48 weeks in study 1423 and up to 96 weeks in study 1540, or until the patient had unacceptable toxicity or confirmed disease progression. The studies were ongoing at the time of last sample collection (September 6, 2017 for study 1423 and October 6, 2017 for study 1540).
The 350 mg Q3W dose regimen was initially proposed on the basis of preliminary calculations of cemiplimab exposure in a typical patient (body weight 75 kg). In the preliminary evaluation, cemiplimab exposure generated by three Q2W cemiplimab doses of 3 mg/kg, the RP2D, over a typical 6-week treatment period was first calculated. Then the estimated cemiplimab exposure over 6 weeks was used to identify a Q3W dose administered twice in the same 6-week timeframe. The cemiplimab exposure generated by the preliminarily selected 350 mg Q3W dose regimen was subsequently simulated using PopPK modeling and simulations and then confirmed by observed data in patients from study 1540.
PopPK Model Development and Simulations
The PopPK model was developed in three stages by non-linear mixed-effects modeling using NONMEM® (7.4, ICON Development Solutions, Ellicott City, Maryland, USA) [17]. At stage 1, a base model describing the PK of cemiplimab without covariate considerations was created. Various base model structures were evaluated, including linear two-compartment models with an empirical non-linear function describing time-varying change in cemiplimab clearance. At stage 2, a full covariate model, incorporating all pre-specified covariate parameters, was developed. The assessed covariates included body weight, albumin concentration, creatinine concentration, creatinine clearance, total bilirubin concentration, tumor type (CSCC, NSCLC, and others; mCSCC vs. laCSCC), and disease characteristics (Eastern Cooperative Oncology Group score 0, 1, or 2) at baseline. At stage 3, a final PopPK model retaining the covariates that improved goodness-of-fit was established. The PopPK model was validated by bootstrap methods and visual predictive checks.
Population-predicted post hoc estimates of concentration–time profiles upon multiple dosing of cemiplimab at 3 mg/kg Q2W and 350 mg Q3W were generated on the basis of the study patient population (505 patients; body weight range 30.9–156 kg; median 76.1 kg) using the final PopPK model with all retained covariates for cemiplimab. In the PopPK model, steady state was considered reached after 16 weeks of treatment. Exposure metrics at steady state (Cmax,ss, Cmin,ss, and AUC6wk,ss) were reported. AUC6wk,ss was estimated over a 6-week treatment period at steady state to directly compare cemiplimab exposure across the two dosing regimens over a similar treatment period. The evaluated 6-week treatment period at steady state covered three Q2W doses and two Q3W doses of cemiplimab. These post hoc estimates were compared in terms of overall exposure (mean values) and variability in exposure (standard deviation) and were used to describe the effect of body weight on cemiplimab exposure with 3 mg/kg Q2W versus 350 mg Q3W dose regimens. In addition, simulations over a 24-week cemiplimab administration period in 2000 typical patients with advanced malignancies (body weight range 30.9–156 kg; median 76.1 kg) were performed to generate the full cemiplimab concentration–time profiles for the two dose regimens.
Confirmation of Fixed Dose Selection
Upon selection of the 350 mg Q3W dose regimen on the basis of PopPK modeling and simulations, as described above, a third group of patients with mCSCC in study 1540 were dosed at 350 mg Q3W after completion of enrollment in the first two groups (both dosed at 3 mg/kg Q2W). As such, additional cemiplimab concentration data became available by October 10, 2018 in 53 patients who received cemiplimab 350 mg Q3W. An overlay of the individual observed cemiplimab concentration data in 51 patients who received cemiplimab 350 mg Q3W on the simulated concentration–time profiles of the same dose regimen was performed to confirm accuracy of the PopPK modeling and simulations approach for dose selection. Data from two patients who had received a lower first dose of cemiplimab (216 mg and 240 mg, respectively) were excluded.
Compliance with Ethics Guidelines
Studies were conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation Guidelines for Good Clinical Practice. The study protocol was approved by ethics committees (committee names and reference numbers are available in the Supplementary Material). Informed consent was obtained from all individual participants included in the study. This article does not contain any studies with animals performed by any of the authors.
Results
PopPK Model
A linear two-compartment PopPK model with zero-order IV infusion rate and first-order elimination rate described the PK of cemiplimab well. Several covariates, including baseline body weight, body mass index, albumin and IgG concentrations in serum, significantly improved the model (p < 0.01). However, the effect of these covariates on cemiplimab concentrations was found to be not clinically relevant (impact of covariates on cemiplimab exposure < 20%; within the typical observed PK variability of approximately 30%). The effect of body weight is further described below on the basis of simulated cemiplimab concentrations. The PopPK model characteristics, including the individual error model, and the individual PK parameters, are described in detail in a separate publication [17]. Key post hoc PK parameters [mean (coefficient of variation)] of cemiplimab in 505 patients at steady state predicted by the final PopPK model included a volume of distribution of 5.20 L (24.3%) and an elimination half-life of 19.2 days (29.5%). Cemiplimab clearance decreased by 34.6% from 0.33 L/day (40.0%) after the first dose to 0.21 L/day (39.5%) at steady state (Table 1).
Simulated Cemiplimab Concentrations Supporting Fixed Dose Selection
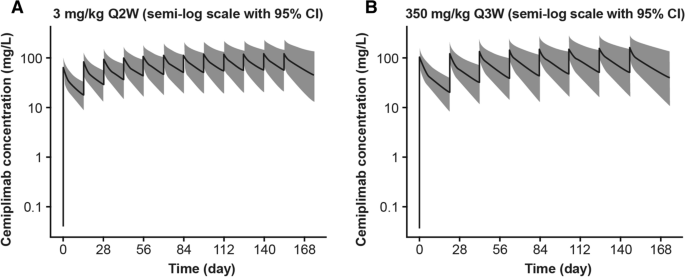
Simulated cemiplimab concentration–time profiles in 2000 typical patients indicate that at steady state cemiplimab concentrations in serum were similar with 350 mg Q3W versus 3 mg/kg Q2W dose regimens (Fig. 1). The mean values for post hoc estimates of cemiplimab exposure in 505 patients after the first dose and at steady state are presented in Table 2. As expected from the difference in dose regimens (dose level and frequency), while cemiplimab concentrations after the first dose were slightly different, they were similar at steady state (AUC6wk,ss, Cmax,ss, and Ctrough,ss). The variability in exposure, as indicated by standard deviations of exposure metrics at steady state, was also similar with the two dose regimens. The frequency distribution of post hoc estimates of AUC6wk,ss at 3 mg/kg Q2W and 350 mg Q3W (Fig. 2) indicated that weight-based dosing did not reduce inter-patient variability in cemiplimab exposure, supporting the preference for a fixed dose regimen. The 350 mg Q3W and 3 mg/kg Q2W dose regimens were expected to generate similar cemiplimab exposures, as demonstrated by overlapping frequency distributions, similar median AUC6wk,ss values, and similar range in exposure.
Simulated cemiplimab concentration–time profiles in patients with advanced malignancies (n = 2000). Plots show the median (black line) and 95% CI (gray area) of simulated cemiplimab concentration–time profiles from 2000 patients with advanced malignancies. CI confidence interval, Q2W every 2 weeks, Q3W every 3 weeks
Distribution of post hoc cemiplimab AUC6wk,ss estimates in patients with advanced malignancies (n = 505). Plot shows the median (dashed line) and density (shaded area) of cemiplimab AUC6wk,ss distributions. AUC6wk,ss area under cemiplimab concentration–time curve over 6 weeks at steady state, IV intravenous, Q2W every 2 weeks, Q3W every 3 weeks
The effect of body weight on cemiplimab exposure with the two dose regimens was described by post hoc estimates of cemiplimab exposure by baseline body weight in 505 patients across the full body weight range of 30.9–156 kg (Fig. 3). Patients with higher body weight showed a trend of higher cemiplimab exposure with 3 mg/kg Q2W; the trend was reversed for 350 mg Q3W. The same trend was shown by post hoc estimates of cemiplimab exposure at extreme body weights of 2.5th percentile (47.7 kg) and 97.5th percentile (117 kg) in Table 3.
Observed Cemiplimab Concentration Data Supporting Fixed Dose Selection
An overlay of individual observed cemiplimab concentrations in 51 patients who received cemiplimab 350 mg Q3W and simulated concentration–time profiles from 2000 patients dosed with cemiplimab 350 mg Q3W showed comparable cemiplimab exposure (Fig. 4), demonstrating the robustness of the PopPK model in predicting cemiplimab exposure to support dose selection. In summary, similarity in cemiplimab exposure at steady state was confirmed by observed data at 3 mg/kg Q2W and 350 mg Q3W in patients with advanced CSCC in study 1540 (Table 4).
Overlay of observed and simulated cemiplimab concentration–time profiles at 350 mg Q3W. Plot shows the median (black line) and 95% CI (gray area) of simulated cemiplimab concentration–time profiles in 2000 patients with advanced malignancies overlaid with observed data (dots) from patients with advanced CSCC in study 1540. Only individual PK data from patients who were compliant with the cemiplimab 350 mg Q3W dosing regimen are shown. Data from 51 out of 53 patients in study 1540 group 3 are presented in this figure; data from 2 patients who had received a lower first dose of cemiplimab (216 mg and 240 mg, respectively) were excluded. CI confidence interval, CSCC cutaneous squamous cell carcinoma, PK pharmacokinetics, Q3W every 3 weeks.
Discussion
On the basis of cemiplimab PK characteristics and supported by PopPK modeling and simulations, the fixed 350 mg Q3W dose regimen of cemiplimab was selected to maintain cemiplimab concentration in serum similar to the initial weight-based 3 mg/kg Q2W RP2D. These PopPK modeling and simulations results, confirmed by observed cemiplimab concentration in serum data in 51 patients with mCSCC from study 1540, supported approval of the fixed cemiplimab 350 mg Q3W dose regimen by the US FDA (cemiplimab-rwlc) and the European Commission [2, 3].
Cemiplimab potently blocks the interactions of PD-1 with PD-L1 and PD-L2 [1]. This mechanism of action does not depend on direct engagement of cemiplimab with tumor cells; thus, substantial differences in PK are not expected across different tumor types. Indeed, for both the 3 mg/kg Q2W and 350 mg Q3W dose regimens in this study, the cemiplimab concentration data were similar in the overall analysis set of patients with advanced malignancies and the subset of patients with advanced CSCC. Similarly, a study of pembrolizumab using pooled data from three clinical trials in advanced melanoma, NSCLC, and other solid tumor types showed that pembrolizumab concentrations were similar across multiple oncology indications [18]. A PopPK analysis for nivolumab indicated that tumor type and burden had significant, but not clinically relevant, effect on nivolumab clearance [19].
In study 1423, cemiplimab exposure and variability in exposure were similar among patients with advanced malignancies dosed with cemiplimab 200 mg Q2W (n = 20) versus a larger patient population dosed with cemiplimab 3 mg/kg Q2W. In the present study, cemiplimab 350 mg Q3W provided comparable exposure and inter-patient variability in exposure to 3 mg/kg Q2W. These results are consistent with those from a review of dosing strategies for mAbs, which showed that the difference of variability in exposure between weight-based and fixed dosing was generally less than 20% [6]. These results are also consistent with findings from a dosing strategy study of pembrolizumab in patients with cancer, in which fixed (200 mg Q3W) and weight-based (2 mg/kg Q3W) dose regimens were both found appropriate [9]. A similar dosing strategy was reported for nivolumab with a switch from 3 mg/kg Q2W to 240 mg Q2W [20]. A review of fixed versus weight-based dosing in multiple disease areas, including oncology, showed that fixed and weight-based dosing approaches performed similarly across the 12 mAbs investigated [7]. Furthermore, previous studies of anti–PD-1 therapies demonstrated a considerable therapeutic margin, as evidenced by relatively flat exposure–response relationships, for both clinical efficacy and safety, over a wide dosing range, providing flexibility in dose selection [12, 13]. In addition to changing from weight-based to fixed dose, our optimization also extended the dosing frequency from Q2W to Q3W, with the intention to provide further convenience to patients and physicians to encourage treatment compliance.
In this study, similar overall variability in cemiplimab exposure at 350 mg Q3W and 3 mg/kg Q2W was shown by PopPK simulations in patients with extreme body weights and across the full body weight range of 30.9–156 kg. As such, the lower-than-average exposure in patients with high body weight at 350 mg Q3W was comparable to the lower-than-average exposure in patients with low body weight at 3 mg/kg Q2W. The lower-than-average cemiplimab exposure with either dose was insignificant and was therefore expected to have minimal impact on clinical efficacy, considering that clinical efficacy was observed at a dose level as low as 1 mg/kg Q2W in study 1423 and the exposure–response relationships are generally flat in anti–PD-1 therapies including cemiplimab over the concentration range studied [2, 12, 13]. Similarly, the higher-than-average cemiplimab exposure in patients with low body weight at 350 mg Q3W was comparable to the higher-than-average exposure in patients with high body weight at 3 mg/kg Q2W. The safety profile of cemiplimab in patients with higher-than-average cemiplimab exposure with either dose was not expected to show marked differences comparing to that in patients with lower exposure, considering that no dose-limiting toxicities were observed at a dose level as high as 10 mg/kg Q2W in study 1423 and the exposure–response relationships over the studied concentration range are generally flat for anti–PD-1 therapies including cemiplimab [2, 12, 13]. Indeed, subsequent results in patients with mCSCC dosed with cemiplimab 350 mg Q3W in group 3 of study 1540 demonstrated antitumor activity and a safety profile comparable to those in patients in group 1 with mCSCC and in group 2 with laCSCC that received cemiplimab 3 mg/kg Q2W [4, 21,22,23].
The validation of results generated by PopPK modeling and simulations in this study was limited by the availability of data from clinical studies and can be further improved as more data from ongoing cemiplimab studies become available.
Conclusions
This study outlined the cemiplimab IV dosing strategy to move from the body weight-based 3 mg/kg Q2W dosing in the FIH study to the extended fixed 350 mg Q3W dosing based on similarity in exposure, as demonstrated by PopPK modeling and simulations and confirmed by a limited dataset from patients with mCSCC in the 350 mg Q3W dose group in study 1540. This similarity in cemiplimab exposure, together with clinical efficacy and safety findings, supported approval of cemiplimab 350 mg Q3W by the US FDA (cemiplimab-rwlc) and by the European Commission for treatment of patients with mCSCC or laCSCC who are not candidates for curative surgery or curative radiation. The cemiplimab IV 350 mg Q3W dose regimen is being further investigated in multiple indications.
References
Burova E, Hermann A, Waite J, et al. Characterization of the anti-PD-1 antibody REGN2810 and its antitumor activity in human PD-1 knock-in mice. Mol Cancer Ther. 2017;16(5):861–70.
Regeneron Pharmaceuticals, Inc. LIBTAYO® [cemiplimab-rwlc] injection full US prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761097s007lbl.pdf. 2021. Accessed February 23, 2021.
European medicines agency. LIBTAYO® EPAR. https://www.ema.europa.eu/en/medicines/human/EPAR/libtayo. 2019. Accessed February 23, 2021.
Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379(4):341–51.
Fairman D, Narwal R, Liang M, et al. Pharmacokinetics of MEDI4736, a fully human anti-PD-L1 monoclonal antibody, in patients with advanced solid tumors. J Clin Oncol. 2014;32(15_suppl):2602.
Bai S, Jorga K, Xin Y, et al. A guide to rational dosing of monoclonal antibodies. Clin Pharmacokinet. 2012;51(2):119–35.
Wang DD, Zhang S, Zhao H, Men AY, Parivar K. Fixed dosing versus body size-based dosing of monoclonal antibodies in adult clinical trials. J Clin Pharmacol. 2009;49(9):1012–24.
Zhang S, Shi R, Li C, Parivar K, Wang DD. Fixed dosing versus body size-based dosing of therapeutic peptides and proteins in adults. J Clin Pharmacol. 2012;52(1):18–28.
Freshwater T, Kondic A, Ahamadi M, et al. Evaluation of dosing strategy for pembrolizumab for oncology indications. J Immunother Cancer. 2017;5(1):43.
Ogungbenro K, Patel A, Duncombe R, Nuttall R, Clark J, Lorigan P. Dose rationalization of pembrolizumab and nivolumab using pharmacokinetic modeling and simulation and cost analysis. Clin Pharmacol Ther. 2018;103(4):582–90.
Food and Drug Administration. E4 dose-response information to support drug registration. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/e4-dose-response-information-support-drug-registration. 1996. Accessed February 23, 2021.
Feng Y, Wang X, Bajaj G, et al. Nivolumab exposure-response analyses of efficacy and safety in previously treated squamous or nonsquamous non-small cell lung cancer. Clin Cancer Res. 2017;23(18):5394–405.
Patnaik A, Kang SP, Rasco D, et al. Phase I study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res. 2015;21(19):4286–93.
Sherwin CMT, Kiang TKL, Spigarelli MG, Ensom MHH. Fundamentals of population pharmacokinetic modelling. Clin Pharmacokinet. 2012;51(9):573–90.
Williams PJ, Ette EI. The role of population pharmacokinetics in drug development in light of the Food and Drug Administration’s ‘guidance for industry: population pharmacokinetics.’ Clin Pharmacokinet. 2000;39(6):385–95.
Food and Drug Administration. Guidance document: population pharmacokinetics. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/population-pharmacokinetics. 1999. Accessed February 23, 2021.
Yang F, Paccaly AJ, Rippley R, Davis J, DiCioccio A. Selection of fixed dose 350 mg every 3 weeks (Q3W) cemiplimab (anti-PD-1) in patients with advanced malignancies based on population pharmacokinetics (PopPK) modelling. ACoP10, Orlando FL, ISSN: 2688–3953. 2019; Vol 1: S-004. https://www.go-acop.org/assets/ACoP10/documents/ACoP10%20Combined%20Abstracts_2019.pdf. Accessed February 23, 2021.
Ahamadi M, Freshwater T, Prohn M, et al. Model-based characterization of the pharmacokinetics of pembrolizumab: a humanized anti-PD-1 monoclonal antibody in advanced solid tumors. CPT Pharmacometrics Syst Pharmacol. 2017;6(1):49–57.
Bajaj G, Wang X, Agrawal S, Gupta M, Roy A, Feng Y. Model-based population pharmacokinetic analysis of nivolumab in patients with solid tumors. CPT Pharmacometrics Syst Pharmacol. 2017;6(1):58–66.
Zhao X, Suryawanshi S, Hruska M, et al. Assessment of nivolumab benefit–risk profile of a 240-mg flat dose relative to a 3-mg/kg dosing regimen in patients with advanced tumors. Ann Oncol. 2017;28(8):2002–8.
Migden M, Khushalani N, Chang A, et al. Primary analysis of phase 2 results of cemiplimab, a human monoclonal anti-PD-1, in patients (pts) with locally advanced cutaneous squamous cell carcinoma (laCSCC). J Clin Oncol. 2019;37:suppl; abstr 6015.
Guminski AD, Lim AM, Khushalani NI, et al. Phase 2 study of cemiplimab, a human monoclonal anti-PD-1, in patients (pts) with metastatic cutaneous squamous cell carcinoma (mCSCC; group 1): 12-month follow-up. J Clin Oncol. 2019;37:suppl; abstr 9526.
Rischin D, Lim AM, Schmults C, et al. Phase 2 study of 2 dosing regimens of cemiplimab, a human monoclonal anti-PD-1, in metastatic cutaneous squamous cell carcinoma (mCSCC). Ann Oncol. 2019;30(suppl_5):v533–63.
Acknowledgements
The authors would like to thank the patients, their families, all other investigators, and all investigational site members involved in this study. The authors would also like to thank Robert Charnas, a former employee of the study sponsor, who reviewed and provided editorial comments on the manuscript.
Funding
The study funding, including Rapid Service and Open Access Fees for this publication, was provided by Regeneron Pharmaceuticals, Inc. and Sanofi.
Medical Writing, Editorial and Other Assistance
Medical writing support under the direction of the authors was provided by Bu Reinen, PhD, of Prime (Knutsford, UK) and funded by Regeneron Pharmaceuticals, Inc. and Sanofi according to Good Publication Practice guidelines.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
The sponsor was involved in the study design, collection, analysis, and interpretation of data, as well as data checking of information provided in the manuscript. The authors had unrestricted access to study data, were responsible for all content and editorial decisions, and received no honoraria related to the development of this publication.
Disclosures
Michael R. Migden: honoraria and travel expenses from Regeneron Pharmaceuticals, Inc., Sanofi, Novartis, Genentech, Eli Lilly, and Sun Pharma; and institutional research funding from Regeneron Pharmaceuticals, Inc., Novartis, Genentech, and Eli Lilly. Kyriakos P. Papadopoulos: institution research funding (START) from Regeneron Pharmaceuticals, Inc., Merck, Medimmune, Merck KGaA, and Mabspace Biosciences. Danny Rischin: institutional research grant and funding from Regeneron Pharmaceuticals, Inc., Sanofi, Roche, Merck Sharp and Dohme, Bristol-Myers Squibb, GlaxoSmithKline, and Kura Oncology; uncompensated scientific committee and advisory board support from Regeneron Pharmaceuticals, Inc., Merck Sharp and Dohme, Sanofi, Bristol Myers-Squibb, and GlaxoSmithKline; travel and accommodation from Merck Sharp and Dohme and GlaxoSmithKline. Ronda K. Rippley: employee and shareholder of Regeneron Pharmaceuticals, Inc. at the time this study was conducted; now employee of Constellation Pharmaceuticals. Anne J. Paccaly, Feng Yang, John D. Davis, Israel Lowy, Matthew G. Fury, and Elizabeth Stankevich: employees and shareholders of Regeneron Pharmaceuticals, Inc.
Compliance with Ethics Guidelines
Studies were conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice. The study protocol was approved by ethics committees, a full list of each committee can be found in the Supplementary Material. Informed consent was obtained from all individual participants included in the study. This article does not contain any studies with animals performed by any of the authors.
Data Availability
Qualified researchers may request access to study documents (including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan) that support the methods and findings reported in this manuscript. Individual anonymized participant data will be considered for sharing once the product and indication has been approved by major health authorities (e.g., FDA, EMA, PMDA, etc.), if there is legal authority to share the data and there is not a reasonable likelihood of participant re-identification. Submit requests to https://vivli.org/.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Paccaly, A.J., Migden, M.R., Papadopoulos, K.P. et al. Fixed Dose of Cemiplimab in Patients with Advanced Malignancies Based on Population Pharmacokinetic Analysis. Adv Ther 38, 2365–2378 (2021). https://doi.org/10.1007/s12325-021-01638-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-021-01638-5