Abstract
Introduction
Chronic kidney disease (CKD) may be associated with overt or subclinical hypothyroidism [SCH; defined as elevated serum thyroid-stimulating hormone (TSH) despite normal free thyroxine levels). Although some studies have demonstrated that thyroid replacement therapy may improve renal function in overt hypothyroidism, there is no consensus on its benefits in SCH. Clinical and limited economic outcomes were evaluated in levothyroxine-treated US veterans with CKD + SCH.
Methods
Veterans Health Administration claims data from April 2013 to March 2018 for levothyroxine-treated versus nontreated CKD + SCH patients were compared. Eligible patients with CKD + SCH (≥ 2 elevated TSH values recorded; ≥ 2 normal thyroxine values recorded) had ≥ 1 TSH values recorded during 24-month follow-up, and ≥ 1 estimated glomerular filtration rate (eGFR) measurement during baseline and follow-up. Continuous levothyroxine use (treatment cohort) was required during follow-up. The primary endpoint was eGFR at 6, 12, 18, and 24 months; secondary endpoints included eGFR change from baseline, CKD progression, and length of hospital stay (LOS). Propensity score matching (PSM) was performed.
Results
Of 453 eligible patients, 157 remained in each cohort after PSM. Most were male (96%) and white (88%); mean age was 75 years. No significant differences were observed between cohorts at any time point for eGFR, eGFR change from baseline, or CKD progression. Treated patients had numerically higher mean eGFR at 6 and 12 months, lower proportions of progression to higher CKD stages at 12, 18, and 24 months, and shorter mean all-cause LOS versus nontreated patients (1.92 vs. 3.30 days; P = 0.3483) within the 24-month follow-up period. A significantly shorter mean CKD-related LOS was observed versus nontreated patients (0.11 vs. 1.38 days; P < 0.0001) during the 24-month follow-up.
Conclusion
Levothyroxine use was associated with economic and clinical benefit in some patients with CKD + SCH, despite an absence of overall benefit on eGFR; confirmatory research is needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Chronic kidney disease (CKD), a common and debilitating condition tied to considerable economic burden, can be comorbid with subclinical hypothyroidism [SCH; i.e., elevated serum thyroid-stimulating hormone (TSH) despite normal free thyroxine levels]. |
Although studies outside the US have shown that levothyroxine replacement therapy in CKD + SCH patients is associated with improvements in renal function, US evidence is lacking. |
This study evaluated renal outcomes and hospital length of stay (LOS) in a CKD + SCH US veteran population treated with levothyroxine. |
What was learned from the study? |
Although there was no significant difference between levothyroxine-treated and untreated CKD + SCH patients over 24 months in clinical outcomes [estimated glomerular filtration rate (eGFR) measures; CKD progression], significantly lower LOS for CKD-related reasons and numerically lower all-cause LOS demonstrated a possible economic benefit for levothyroxine. |
Although not statistically significant, CKD + SCH patients who were levothyroxine-treated had numerically lower proportions of progression to higher CKD stages at 12, 18, and 24 months; in a small subset with baseline TSH > 10 mIU/L (n = 25: 15 treated, 10 untreated), a numerically lower proportion of treated patients had CKD progression to higher CKD stages compared with untreated patients. |
Prospective trials or larger retrospective analyses of sufficient sample size may further elucidate these retrospective database analysis findings. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13295942.
Introduction
Chronic kidney disease (CKD) is a debilitating condition affecting more than 37 million adults in the United States, with annual Medicare costs associated with CKD estimated at up to US$84 billion [1]. The National Health and Nutrition Examination Survey (NHANES III), a national sample of the US population, found that approximately 11–23% of CKD patients also have hypothyroidism, and 56% of these patients have subclinical hypothyroidism [SCH; i.e., elevated serum thyroid-stimulating hormone (TSH) with normal thyroxine (T4)] rather than overt hypothyroidism [2]. A recent meta-analysis also reported that the pooled odds ratio of SCH in CKD was 1.37 (95% CI 1.13–1.67, P = 0.000), independent of conventional risk factors in a community-based population [3].
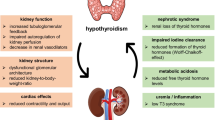
Thyroid hormones have numerous effects on the kidneys, heart, and vascular system, and thyroid dysfunction has a substantial impact on renal function [4,5,6,7,8,9]. Thyroid hormones influence kidney development and growth, sodium and water homeostasis, renal plasma flow, and glomerular filtration rate (GFR) [5, 10, 11]. Decreased thyroid hormone has been shown to result in reduced renal plasma flow and GFR, and impaired urinary concentration and dilution [12]. Reduced GFR and creatinine clearance have been reported in patients with overt hypothyroidism [9, 11, 13,14,15,16], and both have been shown to improve with thyroid replacement therapy, such as levothyroxine [13, 15].
The case for thyroid hormone replacement for SCH in patients with CKD is less clear. Some studies have reported adverse effects associated with SCH in patients with CKD [17], but there is no consensus on the clinical importance or benefit of levothyroxine therapy in patients with SCH and CKD. The 2012 American Association of Clinical Endocrinologists/American Thyroid Association (AACE/ATA) guidelines state that the decision to treat SCH when TSH is < 10 mIU/L should be tailored to the individual patient. The guidelines acknowledge that there are limited clinical cardiovascular outcomes data to support treating patients with SCH when TSH levels are between 4.6 and 9.9 mIU/L, and observe that there is limited but specific information supporting levothyroxine use when TSH is 2.5–4.5 mIU/L in cases of pregnancy [18].
Given the limited evidence, treatment for SCH in the general population remains a controversial issue for which additional data are needed [6, 10]. Furthermore, recent guidelines have recommended against the use of thyroid hormone therapy for patients with SCH because of an absence in benefit of treatment. However, these guidelines suggest the need for future research to explore whether there is an unidentified subgroup of patients that would benefit from treatment of SCH [19]. There is some evidence to suggest that levothyroxine treatment of SCH provides clinical benefit by improving renal function and delaying disease progression in certain subpopulations with CKD in studies conducted outside of the United States [10, 13]. A study by Shin et al. in 2012 reported a significantly higher incidence rate of end-stage renal disease (ESRD) among SCH patients not treated with levothyroxine [10]. Treatment with levothyroxine was associated with a delay in progression to CKD stage 5 or ESRD, as well as overall renal function preservation [10]. Additionally, Hataya et al. reported that eGFR increased rapidly following initiation of levothyroxine in patients with concomitant SCH and CKD; however, the eGFR increase was followed by a plateau [13].
To address the need for more quantitative evidence within the United States on levothyroxine treatment outcomes in patients with CKD and SCH, we evaluated clinical and limited economic outcomes of CKD in patients with SCH treated with levothyroxine compared with patients not treated with levothyroxine.
Methods
Study Design and Data Source
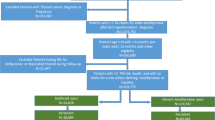
This retrospective observational cohort study used data from the US Veterans Health Administration (VHA) database for the study period of April 1, 2013, through March 31, 2018 (Fig. 1). The VHA database reflects the largest integrated health care system in the United States, providing care at 170 medical centers and 1074 outpatient clinics to more than 9 million veteran enrollees [20]. For 2013–2018, renal function data reported as estimated GFR (eGFR; available at the VHA since 2006) were obtainable from more than 98% of VHA facilities [21]. Through use of the VHA database, this study had access to available laboratory data to identify SCH, in the absence of an International Classification of Diseases (ICD) code, as well as access to laboratory data over the desired follow-up.
Because this retrospective database analysis did not conduct collection, use, or transmittal of identifiable data, institutional review board approval for the study was not required, as it is exempt according to 45CFR46.101(b)(4): Existing Data & Specimens—No Identifiers. Study data meet the requirements of the Health Insurance Portability and Accountability Act of 1996.
Patient Selection
During the patient identification period between April 1, 2014, and March 31, 2016, qualified patients with CKD plus SCH were selected. Included patients had a diagnosis of CKD (defined as ≥ 1 claim for CKD stages 2, 3, or 4, or eGFR 15–89 mL/min/1.73 m2) [22] and a diagnosis of SCH [defined as ≥ 2 elevated TSH values recorded (> 4.12 mIU/L) [18], and ≥ 2 normal total thyroxine (T4; 4–11 µg/dL) values recorded, with evaluations within 14 days of each other during the 12-month baseline period, which began with first elevated TSH value recorded; Fig. 1]. During the 12-month baseline period, patients with eGFR ≥ 90 mL/min/1.73 m2 or any evidence of ESRD/CKD stage 5, renal replacement therapy, or transplant were excluded.
The treatment cohort was required to have one or more prescription claims for AB-rated levothyroxine products during identification, as well as continuous use without discontinuation post-baseline. AB-rated levothyroxine products are defined as US Food and Drug Administration–approved drug products with therapeutic equivalence evaluations [i.e., the drug products are the same formulation (e.g., tablet, capsule, liquid) and have established bioequivalence in the rate and extent of the active ingredients at the site of action]. The AB-rated levothyroxine drug products include Synthroid, Levo-T, Unithroid, or levothyroxine sodium [23]. For the purposes of this trial, continuous use was defined as having the proportion of days covered at ≥ 80% throughout the 24-month follow-up period [or death, transplant, or renal replacement therapy (RRT) during follow-up] and not having a gap between prescriptions greater than the variable grace period based on prescription supply days (e.g., the grace period for a prescription for a 30-day supply is 30 days). Discontinuation was defined as failure to refill the levothyroxine prescription for a length of time greater than the grace period. The index date for the treatment cohort was the date of first levothyroxine prescription fill; for the nontreatment cohort, it was the date of the second confirmatory SCH laboratory test.
Selected patients were aged 18 years or older on the index date, with continuous health plan enrollment 12 months before as well as in the follow-up period (for 24 months, or up to time of death at least 6 months later, transplant, or RRT) (Fig. 1). Additionally, selected patients had at least 6 months of follow-up post-index date, ≥ 1 eGFR laboratory value during baseline and 24-month follow-up, and ≥ 1 additional TSH laboratory value available during the 24-month follow-up. For the treatment cohort, continuous use of levothyroxine (documented by AB-rated levothyroxine prescriptions) during follow-up without discontinuation was required, whereas, for the nontreatment cohort, evidence of no levothyroxine use during follow-up was required. Additional exclusion criteria included levothyroxine use after CKD diagnosis date but before SCH diagnosis (treatment cohort only), pregnancy, thyroid cancer, other nonskin malignancies, or acute kidney injury (within 3 months of the 24-month follow-up).
Study follow-up beyond the minimum 24 months, which was required for primary, secondary, and most other analyses, occurred out to 36 months post-index date (selection criteria similar to those for 24 months) for longer-term evaluations for a single predetermined subgroup analysis and one exploratory endpoint. Long-term exploratory findings are beyond the scope of this report.
Predefined time points and associated data collection windows during the 24-month follow-up for data analyses were at months 6, 12, 18, and 24. Data collection windows included the preceding 3 months for month 6 and the preceding 5 months for months 12, 18, and 24. For all analyses, the value within the collection window closest to the predefined time point was used.
Primary, Secondary, and Exploratory Objectives and Measures
The primary objective was to examine eGFR change over time in treated versus nontreated cohorts during the 24-month follow-up. For patients who had evidence of RRT or renal transplant during the post-baseline study period, the last eGFR observed before the transplant/RRT was included. As indicated above, for patients who had multiple eGFR values within one or more data collection windows, the eGFR value closest to the actual time point was used.
Secondary objectives compared treated versus nontreated cohorts for eGFR change from baseline within 24 months, CKD progression from baseline to 24 months, and healthcare resource utilization (HRU; CKD-related and all cause) within 24 months. If a patient demonstrated CKD progression, defined by progression to a higher (worse) stage of CKD from baseline at 6, 12, 18, and 24 months based on diagnosis or eGFR, the value was carried forward for the subsequent time points; the number and proportion were reported. Those who progressed to RRT or transplant during follow-up were considered in ESRD, with follow-up ending and the value similarly carried forward. All-cause HRU was defined as inpatient length of stay (LOS) within 24 months, whereas CKD-related HRU was defined as CKD-related [based on ICD CKD diagnoses codes] inpatient LOS.
Exploratory analyses included determining within the 24-month follow-up (treated vs. nontreated) the number and proportion of patients who progressed to stage 5 CKD, number and proportion who achieved target TSH measurements (0.45–4.12 mIU/L), changes in mean eGFR over time in patients stratified by baseline TSH (4.12–10 or > 10 mIU/L), and number and proportion who progressed to higher CKD stage also stratified by baseline TSH (4.12–10 or > 10 mIU/L).
A subgroup analysis detailed a priori called for the description in treated patients of clinical (eGFR; CKD progression) and economic outcomes (HRU) for those who achieved TSH target range versus those who did not during 24 months.
Statistical Analysis
Analyses were performed using Statistical Analysis System (SAS), v.9.4 (Cary, NC, USA). All study variables—including patient baseline demographics, clinical characteristics, and follow-up outcomes—were reported descriptively. Numbers and percentages were provided for categorical variables. Means, medians, interquartile ranges, minima, maxima, and standard deviations were provided for continuous variables. Standardized mean differences (SMDs) were calculated for each baseline variable, with an SMD < 10 indicating a good balance between treatment and nontreatment cohorts. Where appropriate, t tests, chi-square tests, and nonparametric tests (e.g., Wilcoxon rank-sum test) were used to test for intercohort differences. Statistical significance was defined as an alpha of 0.05.
Propensity Scoring Matching Analyses
Propensity score matching (PSM) was performed to minimize confounding effects between the treatment and nontreatment cohorts. The propensity score is the probability of receiving the treatment conditional on observed baseline characteristics. This method mimics the design of a randomized controlled trial by balancing patient baseline characteristics to estimate the difference in treatment effect. PSM covariates included age, sex, race, Charlson comorbidity Index, hypertension, medication use (angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, nonsteroidal anti-inflammatory drugs, diuretics), TSH, T4, eGFR, and inpatient LOS (0, 1–8, and ≥ 9 days).
Multivariable Analysis Models
In addition to unadjusted comparisons, multivariable regression models were run in the matched cohorts. The mixed model with repeated measures (MMRM) and generalized linear mixed model (GLMM) were used, respectively, for continuous outcomes (eGFR and change in eGFR) and categorical outcomes (CKD progression, TSH target achieved). The generalized linear model (GLM) was used to evaluate inpatient LOS.
Results
Study Population and Baseline Characteristics
A total of 453 patients (184 and 269 in treatment and nontreatment cohorts, respectively) were eligible after application of selection criteria prior to PSM, and 157 patients remained in each cohort after PSM (Fig. 2).
Study population flow diagram for primary and secondary objectives; AACE American Association of Clinical Endocrinologists, ATA American Thyroid Association, CKD chronic kidney disease, eGFR estimated glomerular filtration rate, PSM propensity score matching; SCH subclinical hypothyroidism, T4 serum thyroxine, TSH serum thyroid-stimulating hormone, VHA Veterans Health Administration
Most patients were male (after PSM: 96.8% vs. 95.5% for treatment vs. nontreatment cohorts, respectively) and elderly (after PSM: mean age, 74.1 vs. 75.2 years). The 3 most common comorbidities at baseline were hypertension (with ≥ 2 out of 3 uncontrolled), diabetes, and diabetic neuropathy. The 2 cohorts were well matched in terms of medication use, TSH levels, and number of patients with stage 2 or 3 CKD after PSM (Table 1).
Primary and Secondary Outcomes
PSM-adjusted comparisons of CKD patients with SCH in the treatment versus nontreatment cohorts indicated no significant differences in mean eGFR values at 6, 12, 18, and 24 months. Although no statistically significant differences were observed, the treated cohort had a numerically higher mean eGFR over year 1; however, this difference diminished during the second year of follow-up (Fig. 3).
Findings from the MMRM model comparing eGFR values showed that eGFR change over time was not significant (coefficient of time: – 0.01; P = 0.8851) and that there was no significant difference of eGFR change over time in treatment versus nontreatment cohorts (coefficient of treatment × time: – 0.15; P = 0.1025).
No significant differences were observed between treatment and nontreatment cohorts for the 2 secondary clinical outcomes. PSM-adjusted analysis did not demonstrate significant differences in eGFR change from baseline to month 24 between treatment and nontreatment groups (Fig. 4). In the MMRM model, comparisons of eGFR change from baseline between cohorts performed at each follow-up time point did not show any significant differences (data not shown). In addition, no significant differences in CKD progression between the 2 groups were found in PSM-adjusted analyses. Although not significant, numerically lower proportions of patients in the treatment cohort progressed to a higher CKD stage at 12, 18, and 24 months (Fig. 5).
The GLMM model for pairwise comparisons showed that patients in the treatment cohort (vs. the nontreatment cohort) had numerically lower odds of progressing to higher CKD stage at 12, 18, and 24 months, but this was not significant (Table 2).
At 24 months, the difference between treatment and nontreatment cohorts for HRU as measured by all-cause inpatient LOS was also not significant (Fig. 6). Although the difference was not significant, the GLM model showed that patients in the treatment cohort had a shorter mean all-cause inpatient LOS compared with the nontreatment cohort (mean: 1.92 vs. 3.30 days, P = 0.3483; Fig. 6), despite having a numerically, but not significantly longer, mean inpatient LOS at baseline (2.71 vs. 1.01 days; Table 1). Furthermore, CKD-related inpatient LOS was significantly shorter in the treatment cohort compared with the nontreatment (mean: 0.11 vs. 1.38 days, P < 0.0001; Fig. 6).
Exploratory analyses were limited in certain cases by low sample size. For example, regardless of treatment status, less than 3% (n = 4 in both groups) of patients progressed to CKD stage 5 during the study. Due to insufficient sample size, no further statistical testing was performed.
Compared with the nontreatment group, a significantly higher proportion of treated patients achieved TSH target range at months 6, 18, and 24 (Fig. 7). The GLMM model with repeated measures for pairwise comparisons confirmed that patients in the treatment cohort (vs. the nontreatment cohort) had higher odds of achieving TSH target at 6, 18, and 24 months (Table 3).
Of the 157 patients in the treatment cohort, 134 (85.4%) achieved TSH within target range by month 24, whereas 23 (14.6%) did not. Treatment cohort TSH target achievers had favorable outcomes compared with the non-TSH target achievers. Significant differences were observed in mean eGFR at 6 (61.6 vs. 45.1, P = 0.0055), 12 (61.9 vs. 50.5, P = 0.0098), and 24 months (60.6 vs. 49.1, P = 0.0261). No significant differences in CKD progression were observed; however, a numeric trend favoring TSH target achievers did emerge. Further analyses in the non-TSH target achievers were not conducted due to the low sample size.
Additionally, in treated TSH target achievers, numerically longer all-cause inpatient LOS (2.05 vs. 1.13 days, P = 0.5528) and CKD-related inpatient LOS (0.13 vs. 0.00, P = 0.0833) were observed, but neither rose to the level of statistical significance.
Lastly, no statistically significant differences in mean eGFR over time were detected in either TSH subgroup (4.12–10 or > 10 mIU/L; results not shown). For patients with TSH > 10 mIU/L at baseline, a numerically higher proportion of untreated patients had CKD progression to a higher stage compared with treated patients, but this was not significant, and no further analysis was conducted due to low sample size (Table 4).
Discussion
This retrospective real-world analysis of PSM-matched patients with CKD and SCH found no significant difference between patients treated with levothyroxine and those not treated with levothyroxine in eGFR value or eGFR change from baseline. Additionally, no statistically significant difference was observed in CKD progression. However, the findings showed that patients treated with levothyroxine trended towards lower odds of CKD progression to a higher stage of disease during the 24-month follow-up compared with nontreated patients, in both the overall patient population, as well as a smaller subset of patients with baseline TSH > 10 mIU/L. The findings also suggested that patients treated with levothyroxine were more likely to achieve TSH target levels, with a trend towards favorable outcomes, when compared with non-TSH target achievers in regard to eGFR values and CKD progression. Finally, significantly lower LOS for CKD-related reasons demonstrated possible economic benefit of levothyroxine treatment.
Considering the current study’s patient population, many of the study’s null results may be attributable to several factors, including differences between male and female populations because the VHA system comprises mostly older men, missing eGFR values that could influence results, and clinical considerations in the aging population (i.e., inherent TSH increases with the normal aging process), which can greatly influence renal dysfunction and study outcomes [11, 13, 24]. Finally, although hypothyroidism remains a chronic condition that should be treated to a TSH target [18], TSH levels in untreated SCH can vary over time—remaining stable, progressing to overt hypothyroidism, or normalizing [25,26,27,28,29,30,31,32]—which could have affected our results. Because elevated TSH may be transient due to intra-individual variation, confirmatory TSH and T4 tests 2 weeks to 3 months after initial values, such as those done in this trial, have been recommended since 2004 [18, 33]. In a review of available data, Karmisholt et al. concluded that, if a repeated TSH value is within 40% of the previous value, “then the result may well be due to random variation.” Consequently, they recommend that the difference between 2 serial TSH tests should be > 40% to suggest a true change in thyroid function [30]. Moreover, in a study looking at the natural history of what is labeled by some as “mild SCH” in 241 women with elevated TSH ≤ 10 mIU/L followed for 5 years, 19.1% (46/241) required levothyroxine therapy, whereas spontaneous normalization was seen in 22.8% (55/241), and only 58.1% (140/241) continued to meet criteria for SCH [34].
Current research suggests that the correlation between hypothyroidism and renal function still remains unclear. A meta-analysis of individual patient data showed that, although decreased thyroid function was shown to correlate with lower eGFR values when evaluated cross-sectionally, there was no association between SCH and deterioration of renal function over time [35]. On the other hand, another recent study evaluating over 15,000 patients found an association between pre-ESRD TSH levels and post-ESRD mortality, with a higher risk of mortality observed among patients with incrementally higher TSH levels [36]. Together, previous research and our findings highlight the need for further evaluation of the link between low thyroid hormone and renal function over time, and whether levothyroxine therapy truly provides clinical benefit.
In terms of the economic aspect, limited published research regarding HRU exists among patients diagnosed with CKD and SCH. Alexander et al. reported in 2009 that the average number of physician visits and hospital admissions increased in patients with later stages of CKD compared with patients with early-stage CKD or no CKD in the United States [37]. Our results with respect to HRU among CKD patients with SCH revealed that patients with CKD and SCH treated with levothyroxine had lower all-cause and CKD-related mean inpatient LOS compared with nontreated patients, suggesting a potential economic benefit of levothyroxine treatment in this population. Future research with more robust economic analysis is needed to gain a better understanding of the true economic impact of initiating levothyroxine treatment for SCH in patients with early stages of CKD, aiming to delay progression.
This study was subject to certain limitations inherent to claims analysis and this specific dataset. Clinical conditions were identified using ICD-9-CM diagnosis codes, which are subject to potential miscoding. The presence of a diagnosis code on a medical claim is not a positive presence of disease, as the diagnosis code may be incorrectly coded or included as rule-out criteria rather than actual disease. In addition, ICD diagnostic codes do not contain the same level of detail as the information provided in patient charts, and some diagnoses are missing from these codes (e.g., there is no ICD-9-CM diagnosis code for SCH). Laboratory value information in claims data may be missing or unavailable. Because this analysis did not use patient charts, some aspects of the patient’s treatment course were outside of the scope of the study. For example, information related to thyroid disease etiology, types of clinical procedures conducted, or the reason behind testing was not available from claims data. Therefore, results of all such claims-based studies should be interpreted accordingly. Specific to this study and the VHA database, eGFR data were not uniformly available for all patients at the same intervals. This is evident from the decrease in sample size at each time point. A linear mixed model was used to account for this limitation. TSH and T4 laboratory values were used to define SCH and are consistent with guidelines from the ATA, AACE, and medical textbooks; however, this does not account for population or age differences. Moreover, because VHA beneficiaries are predominantly older and male, results may not be generalizable to the population of CKD patients with SCH as a whole. Certain information that could have an effect on study outcomes, such as clinical and disease-specific parameters, are not readily available in the VHA database. Finally, the economic outcomes investigated in this study were limited and did not account for medication costs or hospital admissions, two factors that should be included in future studies.
Conclusions
In this retrospective database analysis of US veterans, clinical outcomes such as eGFR values, eGFR changes from baseline, and CKD progression to a higher stage between the treated and nontreated patients were not significantly different. Trends towards lower odds of CKD progression and numerically favorable outcomes in treated patients achieving TSH target compared with non-TSH target achievers point to areas where future research is needed. Significantly lower LOS for CKD-related reasons and a trend towards lower all-cause LOS demonstrate a possible economic benefit of levothyroxine treatment. Although the economic analyses in this study were limited, significant findings for CKD-related LOS suggest future research may be needed to investigate the full economic impact of treating CKD and SCH patients with levothyroxine. Nevertheless, we believe these results provide valuable insights into real-world clinical and economic outcomes of CKD patients with SCH treated with levothyroxine. Future research with larger patient sample sizes is needed to continue to build on the understanding of the clinical and economic impact of levothyroxine treatment in patients with concomitant CKD and SCH.
References
Centers for Disease Control and Prevention. Chronic Kidney Disease Initiative: Chronic Kidney Disease Basics. https://www.cdc.gov/kidneydisease/basics.html. Accessed 26 May 2020.
Lo JC, Chertow GM, Go AS, Hsu CY. Increased prevalence of subclinical and clinical hypothyroidism in persons with chronic kidney disease. Kidney Int. 2005;67:1047–52.
Wang X, Zhao X, Huang X. Association of subclinical thyroid dysfunction with chronic kidney disease: a systematic review and meta-analysis. Endocr Res. 2020;45:41–9.
Asvold BO, Bjoro T, Vatten LJ. Association of thyroid function with estimated glomerular filtration rate in a population-based study: the HUNT study. Eur J Endocrinol. 2011;164:101–5.
Capasso G, De Tommaso G, Pica A, et al. Effects of thyroid hormones on heart and kidney functions. Miner Electrolyte Metab. 1999;25:56–64.
den Hollander JG, Wulkan RW, Mantel MJ, Berghout A. Correlation between severity of thyroid dysfunction and renal function. Clin Endocrinol (Oxf). 2005;62:423–7.
Montenegro J, Gonzalez O, Saracho R, Aguirre R, Gonzalez O, Martinez I. Changes in renal function in primary hypothyroidism. Am J Kidney Dis. 1996;27:195–8.
Verhelst J, Berwaerts J, Marescau B, et al. Serum creatine, creatinine, and other guanidino compounds in patients with thyroid dysfunction. Metabolism. 1997;46:1063–7.
Rhee CM. The interaction between thyroid and kidney disease: an overview of the evidence. Curr Opin Endocrinol Diabetes Obes. 2016;23:407–15.
Shin DH, Lee MJ, Kim SJ, et al. Preservation of renal function by thyroid hormone replacement therapy in chronic kidney disease patients with subclinical hypothyroidism. J Clin Endocrinol Metab. 2012;97:2732–40.
Iglesias P, Bajo MA, Selgas R, Diez JJ. Thyroid dysfunction and kidney disease: an update. Rev Endocr Metab Disord. 2017;18:131–44.
Mariani LH, Berns JS. The renal manifestations of thyroid disease. J Am Soc Nephrol. 2012;23:22–6.
Hataya Y, Igarashi S, Yamashita T, Komatsu Y. Thyroid hormone replacement therapy for primary hypothyroidism leads to significant improvement of renal function in chronic kidney disease patients. Clin Exp Nephrol. 2013;17:525–31.
Iglesias P, Díez JJ. Thyroid dysfunction and kidney disease. Eur J Endocrinol. 2009;160:503–15.
Mooraki A, Broumand B, Neekdoost F, Amirmokri P, Bastani B. Reversible acute renal failure associated with hypothyroidism: report of four cases with a brief review of literature. Nephrology (Carlton). 2003;8:57–60.
van Welsem ME, Lobatto S. Treatment of severe hypothyroidism in a patient with progressive renal failure leads to significant improvement of renal function. Clin Nephrol. 2007;67:391–3.
Afsar B, Yilmaz MI, Siriopol D, et al. Thyroid function and cardiovascular events in chronic kidney disease patients. J Nephrol. 2017;30:235–42.
Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22:1200–35.
Bekkering GE, Agoritsas T, Lytvyn L, et al. Thyroid hormones treatment for subclinical hypothyroidism: a clinical practice guideline. BMJ. 2019;365:l2006.
US Department of Veterans Affairs. About Veterans Health Administration (VHA). Available at: https://www.va.gov/health/aboutVHA.asp. Accessed May 13, 2020.
Centers for Disease Control and Prevention. Chronic Kidney Disease (CKD) Surveillance System: Coreporting of eGFR in VA Facilities Methods. https://nccd.cdc.gov/CKD/detail.aspx?Qnum=Q230. Accessed 13 May 2020.
Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–30.
US Food and Drug Administration. Orange Book: Approved Drug Products With Therapeutic Equivalence Evaluations. https://www.accessdata.fda.gov/scripts/cder/ob/. Accessed 14 May 2020.
Lu Y, Guo H, Liu D, Zhao Z. Preservation of renal function by thyroid hormone replacement in elderly persons with subclinical hypothyroidism. Arch Med Sci. 2016;12:772–7.
Diez JJ, Iglesias P. Spontaneous subclinical hypothyroidism in patients older than 55 years: an analysis of natural course and risk factors for the development of overt thyroid failure. J Clin Endocrinol Metab. 2004;89:4890–7.
Diez JJ, Iglesias P, Burman KD. Spontaneous normalization of thyrotropin concentrations in patients with subclinical hypothyroidism. J Clin Endocrinol Metab. 2005;90:4124–7.
Kabadi UM. “Subclinical hypothyroidism”. Natural course of the syndrome during a prolonged follow-up study. Arch Intern Med. 1993;153:957–61.
Karmisholt J, Andersen S, Laurberg P. Interval between tests and thyroxine estimation method influence outcome of monitoring of subclinical hypothyroidism. J Clin Endocrinol Metab. 2008;93:1634–40.
Karmisholt J, Andersen S, Laurberg P. Variation in thyroid function tests in patients with stable untreated subclinical hypothyroidism. Thyroid. 2008;18:303–8.
Karmisholt J, Andersen S, Laurberg P. Variation in thyroid function in subclinical hypothyroidism: importance of clinical follow-up and therapy. Eur J Endocrinol. 2011;164:317–23.
Karmisholt J, Laurberg P. Serum TSH and serum thyroid peroxidase antibody fluctuate in parallel and high urinary iodine excretion predicts subsequent thyroid failure in a 1-year study of patients with untreated subclinical hypothyroidism. Eur J Endocrinol. 2008;158:209–15.
Meyerovitch J, Rotman-Pikielny P, Sherf M, Battat E, Levy Y, Surks MI. Serum thyrotropin measurements in the community: five-year follow-up in a large network of primary care physicians. Arch Intern Med. 2007;167:1533–8.
Surks MI, Ortiz E, Daniels GH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA. 2004;291:228–38.
Rosario PW, Carvalho M, Calsolari MR. Natural history of subclinical hypothyroidism with TSH ≤10 mIU/l: a prospective study. Clin Endocrinol (Oxf). 2016;84:878–81.
Meuwese CL, van Diepen M, Cappola AR, et al. Low thyroid function is not associated with an accelerated deterioration in renal function. Nephrol Dial Transplant. 2019;34:650–9.
You AS, Sim JJ, Kovesdy CP, et al. Association of thyroid status prior to transition to end-stage renal disease with early dialysis mortality. Nephrol Dial Transplant. 2019;34:2095–104.
Alexander M, Bradbury BD, Kewalramani R, Barlev A, Mohanty SA, Globe D. Chronic kidney disease and US healthcare resource utilization in a nationally representative sample. Am J Nephrol. 2009;29:473–82.
Acknowledgements
Funding
This work and the journal’s Rapid and Open Access Fees were funded by AbbVie Inc. AbbVie participated in the study design, research, data collection, interpretation of data, reviewing, and approval of the publication. No honoraria or payments were made for authorship.
Authorship
Authors have met the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this publication, take complete responsibility for the integrity of the study data and the work as a whole including accuracy in its analyses, and have given their approval for this version to be published. All authors had access to relevant data and participated in the drafting, review, and approval of this publication.
Medical Writing, Editorial, and Other Assistance
Medical writing assistance was provided by Carol L. Mitchell, MD, and Janet E. Matsuura, PhD, of ICON (North Wales, PA) and was funded by AbbVie Inc. Statistical analyses were performed by STATinMED Research and funded by AbbVie Inc.
Disclosures
Yinghui Duan is a full-time salaried employee of AbbVie Inc. and may own stock/options. Ved Gossain declares that he has no conflict of interest. James Hennessey declares that he has no conflict of interest. Seema Soni-Brahmbhatt is a full-time salaried employee of AbbVie Inc. and may own stock/options. Matthew Weir has served on scientific advisory boards for AbbVie Inc.
Compliance with Ethics Guidelines
The authors affirm that this retrospective database analysis did not entail collection, use, or transmittal of identifiable data. Based on 45CFR46.101(b)(4): Existing Data & Specimens—No Identifiers, this study is exempt from the requirement for institutional review board approval. The study is compliant with data security requirements of the Health Insurance Portability and Accountability Act of 1996.
Data Availability
The data set supporting the conclusions in this report is available from the US Veterans Health Administration. However, restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hennessey, J.V., Weir, M.R., Soni-Brahmbhatt, S. et al. Effect of Levothyroxine on Kidney Function in Chronic Kidney Disease with Subclinical Hypothyroidism in US Veterans: A Retrospective Observational Cohort Study. Adv Ther 38, 1185–1201 (2021). https://doi.org/10.1007/s12325-020-01589-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-020-01589-3