Abstract
Introduction
Joubert syndrome (JS) is a recessive disorder characterized by a congenital malformation of the mid-hindbrain and a large spectrum of clinical features including optic nerve morphologic abnormalities. The function of the visual pathways, including the optic nerve, can be objectively evaluated by visual evoked potential (VEP) recordings. Our work aims to employ VEP to evaluate the neural conduction along the visual pathways in JS patients with or without optic nerve morphologic abnormalities (ONMA).
Methods
In this observational and prospective study, 18 children with genetic diagnosis of JS (mean age 8.78 ± 5.87 years) and 17 healthy age-similar control subjects (control group, 9.05 ± 6.02 years) were enrolled.
Based on presence/absence of ONMA at fundus examination, JS patients were divided into two groups: the JS-A group (eight patients with ONMA) and JS-N group (ten patients without ONMA). Following the ISCEV standards, pattern VEPs were recorded in patients and controls in response to 60’ and 15’ checks to obtain a prevalent activation of large or small axons, respectively.
Results
Compared to controls, both the JS-A and JS-N groups showed significant abnormalities in 60’ and 15’ VEP implicit time and amplitude. Only in the JS-N group were values of 15’ VEP implicit significantly correlated with the corresponding values of visual acuity.
Conclusions
Our results suggest that a visual pathways dysfunction (of both large and small axons) detectable by VEP may occur in JS patients regardless of the presence of ONMA. Since clinical trials are envisaged in the near future to address JS-related ocular problems, our results might provide information about the potential usefulness of VEP recordings to assess the efficacy of treatments targeted to improve the visual pathways’ function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Joubert syndrome (JS) is an autosomal recessive disorder characterized by congenital malformation of the mid-hindbrain and a large spectrum of clinical features including optic nerve morphologic abnormalities (ONMA). |
The function of all of the visual pathways, including the optic nerve, can be objectively evaluated by visual evoked potential (VEP) recordings, and, by using appropriate characteristics of visual stimuli (60’ and 15’ checks), it is possible to obtain an objective evaluation of the neural conduction along “large” and “small” axons. |
This observational and prospective study was carried out to evaluate the functional condition of different components (large and small axons) of visual pathways in JS patients with (JS-A) or without (JS-N) ONMA. Our results should provide information about the possible usefulness of VEP recordings in the efficacy assessment of treatments targeted to improve the visual pathway condition. |
What was learned from the study? |
Compared to controls, in both the JS-A and JS-N groups, significant abnormalities of the values of 60’ and 15’ VEP implicit time and amplitude were found. Only in the JS-N group were the values of 15’ VEP implicit time significantly correlated with the corresponding values of visual acuity. |
In JS patients with or without ONMA, a visual pathway dysfunction (in both large and small axons) occurs and is detectable by VEP recordings. VEP methods should be proposed to evaluate the effectiveness of adequate treatment aimed to improve the visual pathway impairment of JS patients. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13084130.
Introduction
In 1969, Dr. Marie Joubert first described four siblings presenting with cognitive impairment, ataxia, episodic tachypnea, eye movement abnormalities and cerebellar vermis agenesis. Subsequently, several patients with Joubert syndrome (JS) were described, presenting a peculiar congenital malformation of the mid-hindbrain associated with neurologic signs and variable multiorgan involvement [1]. The diagnosis of JS is currently based on the presence of the typical “molar tooth sign” on brain imaging, characterized by cerebellar vermian hypoplasia, thickened and horizontalized superior cerebellar peduncles and a deepened interpeduncular fossa [2]. JS can occur as an isolated neurologic disorder or be associated with variable involvement of the retina and optic nerve, kidneys, liver and skeleton [2, 3]. The prevalence of JS has been estimated at approximately 1:100,000 in the US [3] and 0.47:100,000 in Italy [4].
Over 40 genes are known to cause JS with recessive inheritance [5, 6], all encoding for proteins of the primary cilium, a subcellular organelle found on the surface of most cellular types, making JS part of the expanding spectrum of “ciliopathies” [7].
An involvement of the visual system is described in about 30% of JS patients [8], including congenital retinal dystrophy [9,10,11], jerky eye movements, nystagmus and strabismus [1], oculomotor apraxia [12, 13], ptosis and extraocular muscle limitation [14]. Morphologic changes of the optic nerve, such as pallor or coloboma [15, 16], can also be detected, albeit more rarely.
Interestingly, a single study on JS [17] suggested mitochondrial dysfunction similar to that seen in Leber's hereditary optic neuropathy (LHON), a distinct neurodegenerative optic neuropathy in which mitochondrial failure is responsible for optic nerve impairment with consequent severe visual loss [18]. In LHON, idebenone administration was found to improve psychophysical measurements, such as the visual acuity, visual field and color vision [19, 20]. However, these subjective tests do not allow an objective evaluation of the effects of this treatment on optic nerve function.
The function of the visual pathways, including the optic nerve, can be objectively evaluated by visual evoked potential (VEP) recordings, well standardized by ISCEV protocols [21].
By using appropriate characteristics of visual stimuli, it is possible to obtain an objective evaluation of the neural conduction along “large” and “small” axons forming visual pathways [22, 23]. For instance, in LHON, an optic nerve dysfunction, prevalent in the component of the small axons, but also involving the large axons, was detected by using VEP recordings with appropriate visual stimuli [24, 25].
In rare diseases such as JS, the monitoring of visual pathway function over time and/or after specific treatments is a debated topic, because it is not easy to find appropriate tests that can evaluate both visual function and visual pathway function at the same time. Indeed, despite being a sensitive and objective tool to measure visual pathway function, VEPs are generally neglected.
The aim of this study was to evaluate the functional condition of different components (large and small axons) of the visual pathways in JS patients with or without morphologic optic nerve involvement. In addition, since clinical trials aimed at restoring visual function in JS patients are envisaged in the near future, our results might suggest new breakthroughs related to the possible usefulness of VEP recordings to evaluate the efficacy of treatments targeted to improve the visual pathways condition.
Methods
Patients
In this observational and prospective study, 18 Italian children (mean age 8.78 ± 5.87 years) with a neuroradiologically proven “molar tooth sign” were selected (see inclusion criteria below) from a larger cohort of JS patients recruited within the frame of a larger clinical-genetic project on cerebellar and brainstem congenital defects (CBCD).
Seventeen healthy age- and gender-matched (mean age 9.05 ± 6.02 years) control subjects were also enrolled. JS patients and healthy controls were enrolled at the Department of Child Neurology and Child Psychiatry IRCCS C. Mondino, Pavia, and submitted to complete ophthalmologic evaluation (see below) at the Section of Ophthalmology, University of Pavia, IRCCS Fondazione Policlinico San Matteo, Pavia. All subjects underwent a complete diagnostic work-up including a detailed assessment of kidney, liver and heart function.
All patients receiving a neuroradiologic diagnosis of JS underwent an NGS-based molecular analysis of a large panel of ciliary genes, including known JS-related genes [5, 6] and genes responsible for other primary ciliopathies (e.g., skeletal ciliopathies, renal ciliopathies, Bardet-Biedl syndrome, Meckel syndrome, etc.) as well as candidate genes that emerged from whole-exome sequencing studies. Identified pathogenic variants were confirmed by Sanger sequencing, and segregation with the disease in the family was assessed by sequencing family members, when available. Genes responsible for LHON or other non-ciliopathy retinal dystrophies were not included in the panel, as this was mainly aimed at testing patients with Joubert syndrome and other primary ciliopathies.
JS patients and controls had a complete ophthalmologic examination including best corrected visual acuity (BCVA) assessments. Anterior segment evaluation with a slit lamp was performed depending on the patients’ cooperation. Cycloplegic refraction (cyclopentolate 1%) and dilated fundus examination with indirect ophthalmoscopy were performed for each patient.
Since VEP recordings were performed by using a binocular stimulation (see below, VEP assessment), patients were divided in two groups based on the presence (JS-A, n = 8) or absence (JS-N, n = 10) of binocular characteristics of the optic nerve morphologic involvement (i.e., coloboma, pale optic disc head, optic disc head cupping). Since a retinal dysfunctional condition may influence VEP responses [26, 27], the main inclusion criterion was the absence of retinal dystrophies at fundus examination. For JS patients and controls, exclusion criteria were optic media opacities, previous history of optic neuropathy or glaucoma, refractive error < ± 3 equivalent spherical diopters, and concomitant general (i.e., diabetes) or neurologic diseases.
Demographic and genetic data, visual acuity and type of morphologic optic nerve involvement in the control, JS-A and JS-N groups are reported in Table 1.
The research followed the tenets of the Declaration of Helsinki, and the study was approved by local Institutional Review Board (Scientific Committee of Section of Ophthalmology, University of Pavia, IRCCS Fondazione Policlinico San Matteo, Pavia, Italy). Informed consent was obtained from the parents of each child.
Visual Acuity Evaluation
In verbal children, BCVA was assessed by the modified Early Treatment Diabetic Retinopathy Study (ETDRS) Charts (Lighthouse, Low Vision Products, Long Island City, NY, USA) and expressed in logMAR values obtained at the distance of 4, 2, 1 and 0.5 m. In preverbal and nonverbal children, BCVA was measured with Teller acuity cards (TACs), and the values were converted to logMAR.
VEP Assessment
VEP recordings were performed according to ISCEV standard [21] protocols.
Briefly, subjects were seated and adapted to room light in a semi-dark, acoustically isolated room for 10 min in front of the display and surrounded by a uniform field of luminance of five candelas per m2. Pupil diameter was approximately 5 mm. No mydriatic or miotic drugs were used. Visual stimuli were checkerboard patterns (contrast, 80%; mean luminance, 110 cd/m2) generated on a TV monitor and reversed in contrast at the rate of two reversals per second. At the viewing distance of 114 cm, the check edges subtended 60 min (60’) and 15 min (15’) of the visual angle. As suggested by the ISCEV standards, VEPs were recorded in response to 60’ (60’ VEP) and 15’ (15’ VEP) checks to obtain a prevalent activation of large (with 60’ checks) or small (with 15’ checks) axons [22, 25]. The monitor screen subtended 23°. A small fixation target, subtending a visual angle of approximately 0.5° (estimated after considering spectacle-corrected individual refractive errors), was placed at the center of the pattern stimulus. For every VEP acquisition, each patient positively reported that he/she could clearly perceive the fixation target.
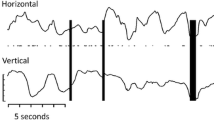
In JS patients and controls, VEPs were recorded by using a binocular stimulation. About this, the ISCEV standards recommend a monocular stimulation for separating the bioelectrical signals of each eye, but it also reports the following: “Monocular stimulation is standard. This may not be practical in infants or other special populations; in such cases binocular stimulation may be used to assess visual pathway function from both eyes” [21]. Since our JS patients can be considered a “special population,” we believe that the binocular stimulation was appropriate with respect to the aim of the study. The monocular stimulation is preferred in patients with different optic nerve conditions between the eyes (i.e., absence of nerve abnormalities in the right eye and coloboma in left eye); in fact, in this case, if a binocular stimulation is performed, the VEP responses are highly related to the normal eye with a negligible contribution of the abnormal eye. To contrast this source of bias, we enrolled selected JS patients based on the presence of the same optic nerve morphologic abnormalities in both eyes or with binocular absence of optic nerve morphologic abnormalities (see inclusion criteria and Table 1).The transient VEP response is characterized by several waves with three subsequent peaks of negative, positive and negative polarity, respectively. In visually normal subjects, these peaks have the following implicit times: 75, 100 and 145 ms (N75, P100 and N145). VEP P100 implicit time (IT) and N75-P100 peak-to-peak amplitude (A) were measured, in milliseconds (ms) and microvolts (µV), respectively, directly on the displayed records by means of a pair of cursors (see Fig. 1).
Examples of visual evoked potential (VEP) responses assessed in one control subject (control #14), in one patient with Joubert syndrome with optic nerve morphologic abnormalities (JS-A #4) and in one patient with Joubert syndrome without optic nerve morphologic abnormalities (JS-N #7); 60’ and 15’ refer to visual stimuli in which each check subtended 60 and 15 min (‘) of the visual arc, respectively. With respect to the control eye, both JS-N and JS-N patients showed VEP responses with delayed P100 implicit times (IT, →) and with a reduction in N75-P100 peak-to-peak amplitude (A, ↕). Relative to JS-A #4, the fundus camera image of the optic nerve morphologic abnormality (coloboma) is also presented
During a recording session, VEPs were recorded at least twice (between 2 to 5 times), and the resulting waveforms were superimposed to check the consistency of results. Based on previous studies [24, 25], we know that intra-individual variability (evaluated by test-retest) is approximately ± 2 ms for VEP P100 IT and approximately ± 0.18 µV for VEP N75-P100 A. During the recording session we considered two successive waveforms “superimposable,” and therefore repeatable, with a difference in ms (for VEP P100 IT) and in µV (for VEP N75-P100 A) less than the above-reported values of intra-individual variability. At times, the first two recordings were sufficient to obtain repeatable waveforms, while other times, further recordings were required (albeit never more than 5 in the cohort of JS patients). For statistical analyses (see below), we considered the VEP P00 IT and N75-P100 A values measured in the recording with the shorter VEP P100 IT.
Statistical Analysis
The Anderson-Darling and Kolmogorov-Smirnov tests were applied to verify that data were normally distributed.
Differences in values of BCVA and VEP parameters (P100 IT and N75-P100 A) between the control and JS groups were evaluated by the one-way analysis of variance (ANOVA). Pearson’s test was used to assess the relationship between the values of BCVA and those of VEP parameters. All statistical analyses were performed using SPSS V.26 (Statistical Package for Social Science IBM), and p < 0.01 was considered as statistically significant.
Results
Visual Acuity Data
On average, a significant (p < 0.01) reduction in BCVA values was observed when data from both the JS-A and JS-N groups were compared to those of controls.
VEP Data
Figure 1 reports examples of VEP responses assessed in one control subject (C #14), in one representative patient with JS and ONMA (JS-A #4) and in one representative patient with JS without ONMA (JS-N #7).
Table 2 presents the mean data of VEP P100 ITs and N75-P100 As (in response to visual stimuli in which each check subtended 60’ and 15’ of the visual arc, respectively) detected in controls, JS, JS-A and JS-N groups and relative statistical analyses among groups.
The VEP results are reported separately as follows:
60’ VEP
In the JS group, P100 ITs and N75-P100 As values were significantly (p < 0.01) increased and reduced, respectively, compared to controls.
Considering individual JS-A patients, the P100 ITs and 75-P100 As were not significantly (p > 0.01) linearly correlated with the corresponding values of BCVA.
On average, compared to controls, in the JS-A group a significant (p < 0.01) increase in P100 ITs values and a significant (p < 0.01) reduction in N75-P100 As values were found.
On average, in the JS-N group a significant (p < 0.01) increase in P100 ITs values and a significant (p < 0.01) reduction in N75-P100 As values were observed with respect to controls. No significant (p > 0.01) differences in mean values of P00 Its and N75-P100 As with respect to those of the JS-N group were found.
In the JS-N group, no significant (p > 0.01) linear correlations between the individual values of P00 ITs and N75-P100 As and the corresponding values of BCVA were observed.
15’ VEP
In the JS group, the mean values of P100 ITs and N75-P100 As were significantly (p < 0.01) increased and reduced, respectively, compared to those of the control group.
Considering JS-A patients, no significant (p > 0.01) linear correlation between individual P100 ITs and 75-P100 As values with the corresponding BCVA values were found.
On average, in the JS-A group, a significant (p < 0.01) increase in P100 ITs values and a significant (p < 0.01) reduction in N75-P100 As values were detected with respect to controls.
On average, with respect to the control group, in the JS-N group a significant (p < 0.01) increase in P100 ITs values and a significant (p < 0.01) reduction in N75-P100 As values were detected. No significant (p > 0.01) differences in the mean values of all VEP parameters with respect to those of JS-N were found.
In the JS-N group, a significant (r = 0.0827, p = 0.003) linear correlation between the individual values of P00 ITs and corresponding values of BCVA was found. No significant correlation between the individual values of N75-P100 A and corresponding values of BCVA was observed.
In the JS group or JS-A and JS-N groups, considering each VEP parameter (P100 IT and N75-P100 A), no significant (p > 0.01) differences were observed between the values obtained with 60’ checks with respect to 15’ checks.
Discussion
The aim of our work was to evaluate the neural conduction along the visual pathways (by VEP recordings) in JS patients with or without optic nerve morphologic abnormalities, with the aim of evaluating this technique as a potential valid and objective method to evaluate the effectiveness of future treatments.
In our study, both JS groups showed VEP P100 ITs significantly delayed and N75-P100 reduced compared to controls, in keeping with the few published studies demonstrating abnormal VEP responses in JS [28,29,30].
It is very interesting that in both the JS-A and JS-N groups, considering each VEP parameter (VEP 100 Its and N75-P100 As), there were no significant differences between the bioelectrical responses obtained with 60’ of visual stimulation and 15’ checks ones. This led us to believe that in JS patients with or without optic nerve morphologic abnormalities, there is a concomitant dysfunction of small and large axons [22, 25].
To explain our VEP findings detected in JS patients, several hypotheses must be considered.
First, there could be a relationship between pathogenic variants in specific JS-related genes and VEP abnormalities. In previous studies in JS, attempts at genotype-phenotype correlations have not been based on VEP recordings [27,28,29], but only on morphologic findings [30, 31]. We acknowledge that the patients’ cohort (n = 18) is too small to draw any meaningful correlations.
A second hypothesis to explain the observed VEP findings is an underlying mitochondrial dysfunction possibly causing optic nerve impairment and consequent visual loss, similar to that seen in LHON [18]. Indeed, this hypothesis has been proposed once about a JS patient [17]. In LHON, several studies reporting VEP abnormalities [24, 25, 32,33,34,35] suggest that a prevalent impairment of small axons (forming the macular-papillo bundle of the optic nerve) may happen. However, different from LHON, in our JS patients we did not detect significant differences between the impaired neural conduction along large axons (delay in 60’ VEP P100IT) and along small axons (delay in 15’ VEP P100IT). This indicates that in JS both large and small axons can be affected, the latter correlating with a reduction of visual acuity exclusively in patients without optic nerve morphologic abnormalities (JS-N group).
A third possibility is demyelination or structural abnormalities of the visual pathways. Maria et al. [36], reporting neuroradiologic data of a cohort of 46 JS patients, suggested an involvement of the myelination process in 33% of cases. This process is crucial for normal neural conduction along visual pathways, and it is well known that in demyelinating neurodegenerative diseases (i.e., multiple sclerosis), there is a delay in neural conduction along the visual pathways detectable by abnormal VEP responses (see Carcelén-Gadea et al. for a review [37]). Thus, it is conceivable that also in JS patients there could be an abnormal myelination of nerve fibers forming the visual pathways, leading to the observed VEP abnormalities.
Regarding the possible selective morphologic abnormalities of visual pathways, postmortem neuropathologic and MRI studies have reported several malformation of multiple brainstem structures, but not of the optic pathways (see Yachnis and Rorke for a review [38]).
Finally, when considering VEP abnormalities in our JS cohort (which reflect a dysfunction of the whole visual pathways, from photoreceptors to the visual cortex [21, 27]), a contribution of possible retinal impairment must be taken in consideration. Indeed, we recently reported retinal dysfunction in JS patients with or without retinal dystrophies, which could be detectable by abnormal electroretinographic responses [39]. Thus, although we enrolled JS patients without retinal dystrophy at fundus examination (see inclusion criteria), a possible contribution of an underlying retinal dysfunction cannot be entirely excluded. Nevertheless, the JS patients enrolled in this study were distinct from those presenting electroretinographic abnormalities [39], and therefore this study does not provide information about a possible relationship between VEP abnormalities and potential retinal dysfunction in the absence of retinal dystrophies at fundus examination.
VEP findings detected in the present cohort of JS patients were overall similar to those observed in patients with other diseases inducing a visual pathway dysfunction (see Creel for a review [27]). Recently, to improve the visual pathway function, several clinical trials have been started in patients with different types of optic neuropathies (LHON, ischemic optic neuropathy, glaucoma) by using treatments with idebenone [19, 20], citicoline [40,41,42,43], coenzyme Q10 [44] or nerve growth factor [45]. In many of these studies [40,41,42,43,44,45], the efficacy of the supplementation treatment was evaluated by measuring the changes of the VEP responses, and it can be foreseen that VEP can be adopted as a useful methodology to evaluate the outcome of future trials aimed at improving visual function in JS patients. A limitation of this study was the small number of JS patients tested (n = 18), which did not allow assessing potential meaningful relationships between VEP findings and variants in specific genes.
Conclusion
In conclusion, our results suggest that in JS patients (with or without optic nerve morphologic abnormalities) there is an impaired neural conduction along the visual pathways, involving both large and small axons. This impairment is detectable by VEP recordings, and this method, associated with psychophysical visual function measures (visual acuity and visual field), may be proposed as a valuable tool for evaluating the effectiveness of adequate treatment targeted to improve the visual pathway function in JS patients.
References
Joubert M, Eisenring JJ, Robb JP, Andermann F. Familial agenesis of the cerebellar vermis. A syndrome of episodic hyperpnea, abnormal eye movements, ataxia, and retardation. Neurology. 1969;19:813–25.
Poretti A, Boltshauser E, Valente EM. The molar tooth sign is pathognomonic for Joubert Syndrome! Pediatr Neurol. 2014;50:e15–6.
Parisi MA. Clinical and molecular features of Joubert syndrome and related disorders. Am J Med Genet C Semin Med Genet. 2009;151C:326–40.
Nuovo S, Bacigalupo I, Ginevrino M, et al. Age and sex prevalence estimate of Joubert syndrome in Italy. Neurology. 2020;94:e797–801.
Stephen J, Vilboux T, Mian L, et al. Mutations in KIAA0753 cause Joubert syndrome associated with growth hormone deficiency. Hum Genet. 2017;136:399–408.
Vilboux T, Doherty DA, Glass IA, et al. Molecular genetic findings and clinical correlations in 100 patients with Joubert syndrome and related disorders prospectively evaluated at a single center. Genet Med. 2017;19:875–82.
Mitchison MM, Valente EM. Motile and non-motile cilia in human pathology: from function to phenotypes. J Pathol. 2017;241:294–309.
Khan AO, Oystreck DT, Seidahmed MZ, et al. Ophthalmic features of Joubert syndrome. Ophthalmology. 2008;115:2286–9.
Dekaban AS. Hereditary syndrome of congenital retinal blindness (Leber), polycystic kidneys and maldevelopment of the brain. Am J Ophthalmol. 1969a;68:1029–37.
Dekaban AS. Familial occurrence of congenital retinal blindness and developmental renal lesions. J Genet Hum. 1969b;17:289–96.
Aicardi J, Castello-Branco ME, Roy C. Joubert’s syndrome. Apropos of 5 cases. Arch Fr Pediatr. 1983;40:625–9.
Tusa RJ, Hove MT. Ocular and oculomotor signs in Joubert syndrome. J Child Neurol. 1999;14:621–7.
Moore AT, Taylor DS. A syndrome of congenital retinal dystrophy and saccade palsy–a subset of Leber’s amaurosis. Br J Ophthalmol. 1984;68:421–31.
Appleton RE, Chitayat D, Jan JE, Kennedy R, Hall JG. Joubert’s syndrome associated with congenital ocular fibrosis and histidinemia. Arch Neurol. 1989;46:579–82.
Laverda AM, Saia OS, Drigo P, Danieli E, Clementi M, Tenconi R. Chorioretinal coloboma and Joubert syndrome: a nonrandom association. J Pediatr. 1984;105:282–4.
Lindhout D, Barth PG, Valk J, Boen-Tan TN. The Joubert syndrome associated with bilateral chorioretinal coloboma. Eur J Pediatr. 1980;134:173–6.
Morava E, Dinopoulos A, Kroes HY, et al. Mitochondrial dysfunction in a patient with Joubert syndrome. Neuropediatrics. 2005;36:214–7.
Carelli V, Ross-Cisneros FN, Sadun AA. Mitochondrial dysfunction as a cause of optic neuropathies. Prog Retin Eye Res. 2004;23:53–89.
La Morgia C, Carbonelli M, Barboni P, Sadun AA, Carelli V. Medical management of hereditary optic neuropathies. Front Neurol. 2014;5:141.
Rudolph G, Dimitriadis K, Boriana Büchner B, et al. Effects of idebenone on color vision in patients with leber hereditary optic neuropathy. J Neuroophthalmol. 2013;33:30–6.
Odom JV, Bach M, Brigell M, et al. ISCEV standard for clinical visual evoked potentials (2009 update). Doc Ophthalmol. 2010;120:111–9.
Harter MR, White CT. Evoked cortical responses to checkerboard patterns: effect of check-size as a function of visual acuity. Electroencephalogr Clin Neurophysiol. 1970;28:48–54.
Parisi V, Scarale ME, Balducci N, Fresina M, Campos EC. Electrophysiological detection of delayed post-retinal neural conduction in human amblyopia. Invest Ophthalmol Vis Sci. 2010;51:5041–8.
Ziccardi L, Sadun F, De Negri AM, et al. Retinal function and neural conduction along the visual pathways in affected and unaffected carriers with Leber’s hereditary optic neuropathy. Invest Ophthalmol Vis Sci. 2013;54:6893–901.
Parisi V, Ziccardi L, Sadun F, et al. Functional changes of retinal ganglion cells and visual pathways in patients with chronic leber’s hereditary optic neuropathy during one year of follow-up. Ophthalmology. 2019;126:1033–44.
Parisi V, Ziccardi L, Stifano G, Montrone L, Gallinaro G, Falsini B. Impact of regional retinal responses on cortical visually evoked responses: multifocal ERGs and VEPs in the retinitis pigmentosa model. Clin Neurophysiol. 2010;121:380–5.
Creel DJ. Visually evoked potentials. Handb Clin Neurol. 2019;160:501–22.
Toma C, Ruberto G, Marzi F, et al. Macular staphyloma in patients affected by Joubert syndrome with retinal dystrophy: a new finding detected by SD-OCT. Doc Ophthalmol. 2018;137:25–36.
Shawkat FS, Harris CM, Taylor DS, Kriss A. The role of ERG/VEP and eye movement recordings in children with ocular motor apraxia. Eye (Lond). 1996;10:53–60.
Hodgkins PR, Harris CM, Shawkat FS, et al. Joubert syndrome: long-term follow-up. Dev Med Child Neurol. 2004;46:694–769.
Brooks BP, Zein WM, Thompson AH, et al. Joubert syndrome: ophthalmological findings in correlation with genotype and hepatorenal disease in 99 patients prospectively evaluated at a single center. Ophthalmology. 2018;125:1937–52.
Salomão SR, Berezovsky A, Andrade RE, Belfort R Jr, Carelli V, Sadun AA. Visual electrophysiologic findings in patients from an extensive Brazilian family with Leber’s hereditary optic neuropathy. Doc Ophthalmol. 2004;108:147–55.
Ziccardi L, Parisi V, Giannini D, et al. Multifocal VEP provide electrophysiological evidence of predominant dysfunction of the optic nerve fibers derived from the central retina in Leber’s hereditary optic neuropathy. Graefes Arch Clin Exp Ophthalmol. 2015;253:1591–600.
Sharkawi E, Oleszczuk JD, Holder GE, Raina J. Clinical and electrophysiological recovery in Leber hereditary optic neuropathy with G3460A mutation. Doc Ophthalmol. 2012;125:71–4.
Jarc-Vidmar M, Tajnik M, Brecelj J, et al. Clinical and electrophysiology findings in Slovene patients with Leber hereditary optic neuropathy. Doc Ophthalmol. 2015;130:179–87.
Maria BL, Hoang KB, Tusa RJ, et al. “Joubert syndrome” revisited: key ocular motor signs with magnetic resonance imaging correlation. J Child Neurol. 1997;120:423–30.
Carcelén-Gadea M, Quintanilla-Bordás C, Gracia-García A, et al. Functional and structural changes in the visual pathway in multiple sclerosis. Brain Behav. 2019;9:e01467.
Yachnis AT, Rorke LB. Neuropathology of Joubert syndrome. J Child Neurol. 1999;14:655–9.
Ruberto G, Parisi V, Bertone C, et al. Electroretinographic assessment in Joubert Syndrome: a suggested objective method to evaluate the effectiveness of future targeted treatment. Adv Ther. 2020;37:3827–38.
Parisi V, Coppola G, Centofanti M, et al. Evidence of the neuroprotective role of citicoline in glaucoma patients. Prog Brain Res. 2008;173:541–54.
Parisi V, Centofanti M, Ziccardi L, et al. Treatment with citicoline eye drops enhances retinal function and neural conduction along the visual pathways in open angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 2015;253:1327–40.
Parisi V, Coppola G, Ziccardi L, Gallinaro G, Falsini B. Cytidine-5’-diphosphocholine (Citicoline): a pilot study in patients with non-arteritic ischaemic optic neuropathy. Eur J Neurol. 2008;15:465–74.
Parisi V, Barbano L, Di Renzo A, Coppola G, Ziccardi L. Neuroenhancement and neuroprotection by oral solution citicoline in non-arteritic ischemic optic neuropathy as a model of neurodegeneration: A randomized pilot study. PLoS ONE. 2019;14:e0220435.
Parisi V, Centofanti M, Gandolfi S, et al. Effects of coenzyme Q10 in conjunction with vitamin e on retinal-evoked and cortical-evoked responses in patients with open-angle glaucoma. J Glaucoma. 2014;23:391–404.
Lambiase A, Aloe L, Centofanti M, et al. Experimental and clinical evidence of neuroprotection by nerve growth factor eye drops: implications for glaucoma. PNAS. 2009;106:13469–74.
Acknowledgements
We thank the participants of the study. The contribution of the Bietti Foundation to this study was supported by the Italian Ministry of Health and Fondazione Roma.
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Giulio Ruberto, Vincenzo Parisi, Chiara Bertone, Sabrina Signorini, Mauro Antonini, Enza Maria Valente, Federica Manzoni, Valentina Serpieri and Riccardo Fausto declare that they have no conflict of interest. Luciano Quaranta is a member of the journal’s Editorial Board.
Compliance with Ethics Guidelines
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the local Institutional Review Board (Scientific Committee of Section of Ophthalmology, University of Pavia, IRCCS Fondazione Policlinico San Matteo, Pavia, Italy). Informed consent was obtained from all individual participants included in the study.
Data Availability
All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ruberto, G., Parisi, V., Bertone, C. et al. Visual Evoked Potentials in Joubert Syndrome: A Suggested Useful Method for Evaluating Future Approaches Targeted to Improve Visual Pathways’ Function. Adv Ther 38, 278–289 (2021). https://doi.org/10.1007/s12325-020-01534-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-020-01534-4