Abstract
Calciphylaxis is a deadly, painful disease with a 1-year mortality of up to 50%. The disease is commonly associated with patients with end-stage kidney disease (ESKD), but it can manifest in non-uremic patients as well. In patients who are undergoing dialysis, the incidence of calciphylaxis can range from 0.04% to 4%. The progressive arterial calcification seen in calciphylaxis can affect multiple body organs, including the skin, brain, lungs, and muscle. In cutaneous calciphylaxis, painful and non-healing nodules, plaques, and ulcers may appear, increasing morbidity for patients. Diagnosis can be difficult, and the condition can clinically appear similar to other dermatological diseases, especially in non-uremic patients. Currently, skin biopsy with histological analysis is the most reliable method to help diagnose the condition. In certain cases, the use of medical imaging may be helpful. Treatment of pain in this condition can be difficult and should be multimodal and include wound care as well as modification of risk factors. Analgesic options include opioids, nonsteroidal anti-inflammatory drugs (NSAIDs), as well as analgesic options that are targeted for specific patients. There are currently multiple clinical trials underway that are studying targeted therapies for this condition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Calciphylaxis is a progressive disease with high mortality. |
Treatment of pain must be multimodal and should target the underlying disease etiology, as pain is often associated with the primary disease burden. Any attempt at analgesia and symptomatic management should be coupled with proper wound care and modification of underlying risk factors. |
Various available therapeutic options include parathyroidectomy, sodium thiosulfate, optimization of renal impairment, cessation of offending drugs, and multimodal analgesic regimens. |
Currently, there are multiple clinical trials in progress in order to provide relief to patients with this debilitating disease. |
Digital Features
This article is published with digital features to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12937544.
Introduction
Calciphylaxis is a progressive inflammatory disease in which small- and medium-sized arteries are calcified, leading to thrombotic ischemia and multiple dermatologic manifestations, ranging from small nodules and plaques to necrotic ulceration [1]. The disease is classically seen in patients with end-stage renal disease (ESRD), where levels of calcium and phosphate in the blood exceed their solubility limit and deposit in the walls of arteries [1, 2]. However, the disease can also be seen in non-uremic patients. Among patients on dialysis, the incidence of calciphylaxis ranges from 0.04% to 4%, and studies report that the incidence has been rising over the last decade [3].
Calciphylaxis is a deadly disease, with the mortality at 6 months being 30% and the mortality at 12 months being 50% [4]. In addition to a high mortality, the disease is highly morbid, with many patients experiencing recurrent hospitalizations, severe pain, and non-healing wounds [5]. Cutaneous calciphylaxis refers to the skins lesions that are seen in the disease process, but vascular calcifications and associated complications can present in other organs, such as the skeletal muscle, lungs, eyes, and the brain [5]. Pain in cutaneous calciphylaxis (CP) can be severe and debilitating; proper treatment requires a multi-specialty approach. Additionally, an effective approach at pain treatment must also target the primary disease burden. This review will focus on describing effective ways to control pain in the setting of calciphylaxis and its cutaneous manifestations.
Pathogenesis
Cutaneous calciphylaxis originates in the body primarily as an arteriolar medial calcification [1]. The disease has a high rate of mortality, in the range of 40–80%, and occurs predominantly in patients with renal failure [6]. The biochemical conditions endemic to patients with this disease, including hyperphosphatemia, hypercalcemia, and hyperglycemia, help incite the transformation of vascular smooth muscle cells into osteoblast-like cells, thereby establishing the mechanics for vascular calcification by ectopic bone formation within the vessel walls [3]. Additionally, a relative lack of inhibitors of calcification (matrix γ-carboxyglutamic acid protein, osteoprotegerin) is thought to play a role in pathologic vascular calcification in patients with chronic kidney disease [3].
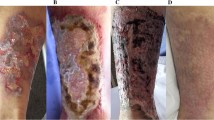
The preceding factors that alter smooth muscle cells promote the narrowing and induration of the affected arteriolar lumens, compromising their structural integrity, and ultimately foster a more conducive environment for thrombotic development. Thrombosis, once manifest, catalyzes the vessel occlusion that leads to the development of ischemic injuries, which in advanced cases leads to dermal necrosis and secondary infections that can promote sepsis. Cutaneous lesions that form include livedo reticularis, plaques, nodules, and ulcers [4]. In the absence of ulceration, the presentation of well-demarcated, erythematous plaques can often mimic cellulitis. The progression of these plaques to an ulcerated state dramatically increases the mortality rate associated with the disease (from 33% to above 80% at 6 months upon development of ulceration) [5].
The major risk factors for calciphylaxis are renal failure as previously mentioned, aberrations in calcium and phosphate homeostasis as in hyperphosphatemia, hypercalcemia, hyper- and hypoparathyroidism, and vitamin K deficiency. Other risk factors that have been shown to predispose patients to calciphylaxis include those that lead to decreased tissue perfusion, as can be seen in patients with obesity and/or diabetes mellitus, and a hypercoagulable state [5].
Renal failure presents consistently in patients with calciphylaxis. A large retrospective review of more than 1000 dialysis patients with calciphylaxis showed that the median time between initiation of dialysis and manifestation of uremic calciphylaxis was 2.5 years [6]. While ESRD is not a necessary pre-requisite condition for calciphylaxis, studies have observed that the rate of development is much lower in non-uremic cases [7]. Vitamin K deficiency is another prominent risk factor due to the vitamin’s role in activating the matrix Gla protein (MGP), which acts as a strong inhibitor of calcification in the vessel walls [2, 8]. ESRD and vitamin K deficiency are commonly found concurrently based on the typical use of warfarin, a vitamin K antagonist, as a component of the treatment paradigm for ESRD. A study found that 40–50% of patients with ESRD and calciphylaxis were taking warfarin at the time of diagnosis [9]. The hypercoagulation risk factor is linked to the increased propensity for development of thrombosis in calcified vessels, accelerating the advanced morbid effects of calciphylaxis on cutaneous tissue. One study showed that 65% of patients with calciphylaxis had at least one hypercoagulable condition, with 20% having two conditions [10].
Patients diagnosed with diabetes mellitus, a disease commonly associated with obesity, have been found to be at higher risk of developing calciphylaxis as well [11]. This relationship can be explained by increased dermal trauma in patients with diabetes as well as the increased propensity for calciphylaxis to target adipose tissue. Studies have shown that calciphylaxis typically occurs on areas of the skin that are frequently and repeatedly subjected to trauma from insulin injections; the risk increases as a function of the number of daily injections around areas of high adipose tissue formation commonly used as injection sites, such as the abdomen and thighs [6]. One possible explanation for the stronger tendency for the disease to manifest in adipose tissue is that adipocytes exposed to high phosphate concentrations can cause vascular smooth muscle cells to calcify in vitro [12].
The specific complications concerning higher incidence of adipose tissue in patients with obesity may extend further beyond phosphate’s role as a calcification catalyst in adipocytes. It has been shown with obesity that the rate of growth of adipose tissue outpaces the capacity for existing capillary density to function effectively and provide requisite metabolic support. This creates the conditions for microcirculation dysfunction from the associated lower perfusion efficacy and resulting local hypoxia [13].
Diagnosis
The diagnosis of calciphylaxis stems from the insidious nature of its progression; the early stages of development, including the medial calcification and subsequent adverse tissue effects, occur alongside severe pain in the afflicted regions in the majority of examined cases. An overwhelming proportion of these patients require opiate-based painkillers to manage the pain [11], and the onset of pain has even been shown to predate any superficial signs of lesions [11, 14].
Even once skin lesions present in a patient, the diagnosis can be difficult to make. In many cases, calciphylaxis will manifest as less conspicuous dermatological abnormalities such as a small papule, plaque, or erosion which can easily be symptomatic of other conditions, such as pyoderma gangrenosum, cellulitis, or ischemic leg ulcers [15, 16]. In these non-uremic cases, histological examination via biopsy is necessary for accurate diagnosis. As detailed before, patients with ESRD are at high risk and therefore clinical diagnosis can be made without a biopsy when these uremic cases present with hardened tender plaques and ulcers in highly predisposed areas with high adipose tissue density. More generally, these initial skin afflictions including indurated plaques and nodules, livedo, or purpura will manifest in groups, bilaterally with a short timeframe of progression to stellate, malodorous ulcers with black eschars [4].
Currently, the best diagnostic technique in the setting of an unclear clinical presentation is a skin biopsy and histological examination [3, 4]. Histologic analysis of the skin may reveal intimal layer fibrosis of blood vessels as well as thrombosis of microvessels in the dermis and subcutaneous tissue [3, 4]. The characteristic feature of calciphylaxis is the diffuse calcification of small capillaries in adipose tissue [3]. Skin biopsy is especially useful in non-uremic cases, where the presentation of skin lesions can mimic other disease processes. However, even in these cases, biopsy may reveal non-specific histological features, furthering complicating the diagnosis. Punch biopsy is a safer approach than an excisional biopsy owing to the decreased likelihood of inducing ulcers through trauma to the compromised areas; specifically, the double-punch technique leverages the initial penetration of the first to gain access to deeper subcutaneous tissue upon which histologic examination is more likely to render accurate judgement of the pathologic features of the disease [3]. The risks attributable to skin trauma and its potential to exacerbate ulceration and subsequent necrosis must be balanced with the risks of foregone diagnosis, or within the spectrum of biopsy techniques, the risk of false negatives. Biopsy of an active lesion is also a viable option, but sampling is preferentially done on the margin of the lesion to avoid necrotic tissue with little diagnostic value [4].
In certain cases, medical imaging can be helpful in making a diagnosis. Specifically, bone scans can discern the presence of subtle calcification features in cases where a biopsy is inappropriate or non-diagnostic. The bone scan works by elucidating abnormalities where the tracer used binds to hydroxyapatite crystals at calcified sites in the dermis and subcutaneous fat [3, 4]. Mammogram and plain radiography are not commonly recommended, as their lower sensitivity to calcification detection make them a suboptimal choice [3, 17].
The current literature shows that serum laboratory investigations yield little in the way of definitive proof in calciphylaxis cases. Although serum calcium phosphate and aluminum levels have specific pathological links to the calcification processes that aggravate the disease, no statistical significance was found in a study examining patients with calciphylaxis against a control group regarding those levels. Additionally, there was no statistical difference in parathyroid hormone (PTH) level between dialysis cases and the control. However, PTH can still play a factor in disease pathogenesis via hyper- or hypoparathyroidism, which can lead to high-turnover bone disease or adynamic bone disease.
Treatment
The timely recognition and treatment of CP necessitates a high index of clinical suspicion, as many medical conditions may mimic CP (Table 1). CP should be suspected in patients with characteristic skin findings, especially in the presence of known risk factors such as renal disease or altered calcium and phosphate homeostasis.
Incomplete understanding of the pathophysiology of CP has hindered the development of specific therapeutic guidelines, leaving much to be desired in terms of high-quality, evidence-based recommendations. Given the high mortality rate and multi-morbidities of patients with CP, it is difficult to acquire patients for randomized controlled clinical trials investigating the disease [18]. Recent or planned clinical studies involving CP are summarized in Table 2. Treatment is largely based upon observational studies and clinical expertise. The mainstay of therapy involves a three-pronged, multidisciplinary approach, as illustrated in Fig. 1.
Early surgical debridement in patients with CP is essential, as surgical debridement is associated with higher 6-month survival when compared to lack of debridement [18,19,20]. Local wound treatment regimens should involve removal of wound debris, application of non-adhesive wound dressings, and utilization of antiseptic or antimicrobial agents as needed [18]. As an adjunctive treatment, hyperbaric oxygen therapy has been shown to achieve 58% wound improvement and 50% wound resolution in uremic calciphylaxis [18, 21]. It is important to promote optimal healing via nutritional management, correction of anemia, and optimization of dialysis parameters [20, 22].
Wound infection is not uncommon in patients with CP and should be suspected when there is increased drainage, pain, or swelling at the site of the wound. Early detection is vital, and treatment is with empiric broad-spectrum antibiotic agents.
In addition to wound management, attention should be given to any risk factors that may contribute to the development of calciphylaxis, such as medications or comorbid conditions. Medications that confer increased risk for calciphylaxis should be ceased or adjusted. Such medications include:
-
Warfarin
-
Vitamin D supplements
-
Calcium supplements
-
Calcium-based phosphate binders
-
Iron supplements
-
Systemic corticoid steroid use
Aberrations in levels of serum of serum calcium, phosphate, and PTH should be addressed. Non-calcium-based phosphate binders (sevelamer hydrochloride, lanthanum carbonate) are preferred over calcium-based binders [5, 18, 23]. Case reports have reported benefit in lowering elevated calcium × phosphate product (Ca × P), but the optimal target product remains debated [24, 25]. For patients with secondary hyperparathyroidism and hyperphosphatemia, cinacalcet should be initiated for PTH levels greater than 300 pg/mL [5]. In a randomized trial of 3883 dialysis patients, risk of calciphylaxis was reduced in patients who received cinacalcet compared to patients receiving placebo (6 versus 18 patients, p = 0.009) [26]. In the presence of hyperparathyroidism, immediate parathyroidectomy may be performed. Routine parathyroidectomy in the absence of hyperparathyroidism is no longer preferred over medical management as the procedure carries risks and survival data are inconclusive [23, 27, 28]. Systemic corticosteroids should not be used as a treatment modality in patients with calciphylaxis, as they can contribute to the progression of calcium and phosphate disarray by initiating bone disease and degradation [15]. In fact, corticosteroid use has been identified as an independent risk factor for calciphylaxis [15].
While it is important to address any underlying risk factors for calciphylaxis, symptomatic pain management should be sought concurrently with medical management. Analgesia is of utmost importance because the pain experienced in CP is frequently disproportionate to the apparent clinical examination and often resistant to opioids [18, 29]. Thus, a broad approach to analgesia is required, with should be tailored to individual patient needs. Achievement of adequate pain relief is challenging because of the complexity and severity of pain, and pain management consultation is often required [29]. In patients with psychological comorbidities, it is important to consider the use of psychotropic agents and anticonvulsants as co-medications to analgesics. Cannabinoids are thought to have antinociceptive properties and may be explored as an alternative method of pain control. Combinations of opioid and ketamine have been shown to be effective especially for refractory pain [29, 30]. Nonsteroidal anti-inflammatory agents may be used in patients without renal impairment. The exact mechanisms behind calciphylaxis pain are not fully understood, but both ischemic and neuropathic components are thought to play a role [29, 31].
Case series and retrospective studies have reported efficacy of sodium thiosulfate (STS) for treating CP. STS is thought to have vasodilatory and chelating properties, and in vitro studies have shown that STS decreases calcification in vascular smooth muscle cells [32, 33]. Because there are no prospective clinical trials investigating STS, evidence supporting its use is limited to retrospective studies [34, 35]. However, a meta-analysis of 856 patients treated with and without STS reported no difference in mortality or wound progression in patients with CP [36].
Patients with CP whose disease remains inadequately controlled even after optimization of calcium, phosphate, and PTH levels and a trial of STS may benefit from second-line therapy. One such therapy is hyperbaric oxygen therapy, which has shown promise in several case reports [21, 37]. Unfortunately, the meta-analysis mentioned above found that found that hyperbaric oxygen therapy did not reduce risk of mortality or wound progression in patients with CP [36]. Bisphosphonates are another systemic therapy that is often used to treat CP, although once again, meta-analysis data showed no reduced risk of mortality [36].
Investigative therapies have been used in patient with treatment-resistant CP including sterile maggot therapy [38], tissue plasminogen activator infusion, vitamin K supplementation, and prednisone [39]. Denosumab is a RANKL inhibitor [40] which has been used to treat hypercalcemia [41]. Experimental studies have shown that denosumab can prevent vascular calcification [42], which could theoretically impede the progression of calciphylaxis, but this finding has not been confirmed in clinical studies [40, 43]. SNF472 is a compound that selectively inhibits hydroxyapatite, which plays a key role in vascular calcification [44]. Previous studies have demonstrated its efficacy in preventing vascular calcification in dialysis patients [44, 45], and it is currently being assessed as a possible therapy for patients with calciphylaxis in phase 2 and 3 trials. All decisions to pursue such therapies should be guided by patient-related risk factors.
Conclusion
Calciphylaxis is a progressive disease with a high morbidity and mortality. Cutaneous lesions manifest in a significant number of patients, such as nodules, plaques, purpura, and ulcers. These cutaneous lesions have the potential to be extremely painful and difficult to treat. Cutaneous calciphylaxis can mimic many other skin conditions and diagnosis can be difficult to make, especially in non-uremic patients. Currently, skin biopsy with histological examination is the best method of diagnosing the disease, with medical imaging being helpful in select cases. Treatment of pain must be multimodal and should target the underlying disease etiology, as pain is often associated with the primary disease burden. Ideally, any attempt at analgesia and symptomatic management should be coupled with proper wound care and modification of underlying risk factors. Currently, there are multiple clinical trials in progress in order to provide relief to patients with this debilitating disease.
References
Wollina U. Update on cutaneous calciphylaxis. Indian J Dermatol. 2013;58(2):87–92. https://doi.org/10.4103/0019-5154.108026.
Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61(6):2210–7. https://doi.org/10.1046/j.1523-1755.2002.00375.x.
Westphal SG, Plumb T. Calciphylaxis. StatPearls. 2020. https://www.ncbi.nlm.nih.gov/pubmed/30085562. Accessed 17 Aug 2020.
Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378(18):1704–14. https://doi.org/10.1056/NEJMra1505292.
Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66(1):133–46. https://doi.org/10.1053/j.ajkd.2015.01.034.
Weenig RH. Pathogenesis of calciphylaxis: Hans Selye to nuclear factor κ-B. J Am Acad Dermatol. 2008;58(3):458–71. https://doi.org/10.1016/j.jaad.2007.12.006.
Chang JJ. Calciphylaxis: diagnosis, pathogenesis, and treatment. Adv Ski Wound Care. 2019;32(5):205–15. https://doi.org/10.1097/01.ASW.0000554443.14002.13.
Nigwekar SU. Calciphylaxis. Curr Opin Nephrol Hypertens. 2017;26(4):276–81. https://doi.org/10.1097/MNH.0000000000000328.
Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27(11):3421–9. https://doi.org/10.1681/ASN.2015091065.
Bajaj R, Courbebaisse M, Kroshinsky D, Thadhani RI, Nigwekar SU. Calciphylaxis in patients with normal renal function: a case series and systematic review. Mayo Clin Proc. 2018;93(9):1202–12. https://doi.org/10.1016/j.mayocp.2018.06.001.
Nigwekar SU, Bloch DB, Nazarian RM, et al. Vitamin K-dependent carboxylation of matrix gla protein influences the risk of calciphylaxis. J Am Soc Nephrol. 2017;28(6):1717–22. https://doi.org/10.1681/ASN.2016060651.
Kramann R, Brandenburg VM, Schurgers LJ, et al. Novel insights into osteogenesis and matrix remodelling associated with calcific uraemic arteriolopathy. Nephrol Dial Transplant. 2013;28(4):856–68. https://doi.org/10.1093/ndt/gfs466.
Brandenburg VM, Kramann R, Rothe H, et al. Calcific uraemic arteriolopathy (calciphylaxis): data from a large nationwide registry. Nephrol Dial Transplant. 2017;32(1):126–32. https://doi.org/10.1093/ndt/gfv438.
el-Azhary RA, Patzelt MT, McBane RD, et al. Calciphylaxis: a disease of pannicular thrombosis. Mayo Clin Proc. 2016;91(10):1395–402. https://doi.org/10.1016/j.mayocp.2016.06.026.
Weenig RH, Sewell LD, Davis MDP, McCarthy JT, Pittelkow MR. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56(4):569–79. https://doi.org/10.1016/j.jaad.2006.08.065.
Chen NX, O’Neill K, Akl NK, Moe SM. Adipocyte induced arterial calcification is prevented with sodium thiosulfate. Biochem Biophys Res Commun. 2014;449(1):151–6. https://doi.org/10.1016/j.bbrc.2014.05.005.
Ye J. Adipose tissue vascularization: its role in chronic inflammation. Curr Diab Rep. 2011;11(3):203–10. https://doi.org/10.1007/s11892-011-0183-1.
Polizzotto MN, Bryan T, Ashby MA, Martin P. Symptomatic management of calciphylaxis: a case series and review of the literature. J Pain Symptom Manag. 2006;32(2):186–90. https://doi.org/10.1016/j.jpainsymman.2006.03.009.
Ghosh T, Winchester DS, Davis MDP, el-Azhary R, Comfere NI. Early clinical presentations and progression of calciphylaxis. Int J Dermatol. 2017;56(8):856–61. https://doi.org/10.1111/ijd.13622.
Hafner J. Calciphylaxis and Martorell hypertensive ischemic leg ulcer: same pattern-one pathophysiology. Dermatology. 2017;232(5):523–33. https://doi.org/10.1159/000448245.
Shmidt E, Murthy NS, Knudsen JM, et al. Net-like pattern of calcification on plain soft-tissueradiographs in patients with calciphylaxis. J Am Acad Dermatol. 2012. https://doi.org/10.1016/j.jaad.2012.05.037.
Erfurt-Berge C, Renner R. Management of patients with calciphylaxis: current perspectives. Chronic Wound Care Manag Res. 2019;6:109–15. https://doi.org/10.2147/cwcmr.s182417.
McCarthy JT, el-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in101 patients with calciphylaxis. Mayo Clin Proc. 2016;91(10):1384–94. https://doi.org/10.1016/j.mayocp.2016.06.025.
Vos T, Abajobir AA, Abbafati C, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390(10100):1211–59. https://doi.org/10.1016/S0140-6736(17)32154-2.
Kaye AD, Novitch MB, Carlson SF, et al. The role of exparel plus meloxicam for postoperative pain management. Curr Pain Headache Rep. 2020;24(3):6. https://doi.org/10.1007/s11916-020-0837-2.
An J, Devaney B, Ooi KY, Ford S, Frawley G, Menahem S. Hyperbaric oxygen in the treatment of calciphylaxis: a case series and literature review. Nephrology. 2015;20(7):444–50. https://doi.org/10.1111/nep.12433.
Jean G, Terrat JC, Vanel T, et al. Calciphylaxis in dialysis patients: to recognize and treat it as soon as possible. Nephrol Ther. 2010;6(6):499–504. https://doi.org/10.1016/j.nephro.2010.04.003.
Russell R, Brookshire MA, Zekonis M, Moe SM. Distal calcific uremic arteriolopathy in a hemodialysis patient responds to lowering of Ca × P product and aggressive wound care. Clin Nephrol. 2002;58(3):238–43. https://doi.org/10.5414/cnp58238.
Don BR, Chin AI. A strategy for the treatment of calcific uremic arteriolopathy (calciphylaxis) employing a combination of therapies. Clin Nephrol. 2003;59(6):463–70. https://doi.org/10.5414/CNP59463.
Perkovic V, Neal B. Trials in kidney disease—time to EVOLVE. N Engl J Med. 2012;367(26):2541–2. https://doi.org/10.1056/NEJMe1212368.
Lal G, Nowell AG, Liao J, Sugg SL, Weigel RJ, Howe JR. Determinants of survival in patients with calciphylaxis: a multivariate analysis. Surgery. 2009;146(6):1028–34. https://doi.org/10.1016/j.surg.2009.09.022.
Kriskovich MD, Holman JM, Haller JR. Calciphylaxis: is there a role for parathyroidectomy? Laryngoscope. 2000;110(4):603–7. https://doi.org/10.1097/00005537-200004000-00012.
Arch-Ferrer JE, Beenken SW, Rue LW, et al. Therapy for calciphylaxis: an outcome analysis. Surgery. 2003;134:941–4. https://doi.org/10.1016/j.surg.2003.07.001.
Sowers KM, Hayden MR. Calcific uremic arteriolopathy: pathophysiology, reactive oxygen species and therapeutic approaches. Oxid Med Cell Longev. 2010;3(2):109–21. https://doi.org/10.4161/oxim.3.2.11354.
Zitt E, König M, Vychytil A, et al. Use of sodium thiosulphate in a multi-interventional setting for the treatment of calciphylaxis in dialysis patients. Nephrol Dial Transplant. 2013;28(5):1232–40. https://doi.org/10.1093/ndt/gfs548.
Udomkarnjananun S, Kongnatthasate K, Praditpornsilpa K, Eiam-Ong S, Jaber BL, Susantitaphong P. Treatment of calciphylaxis in CKD: a systematic review and meta-analysis. Kidney Int Rep. 2019;4(2):231–44. https://doi.org/10.1016/j.ekir.2018.10.002.
Baldwin C, Farah M, Leung M, et al. Multi-intervention management of calciphylaxis: a report of 7 cases. Am J Kidney Dis. 2011;58(6):988–91. https://doi.org/10.1053/j.ajkd.2011.06.022.
Tittelbach J, Graefe T, Wollina U. Painful ulcers in calciphylaxis—combined treatment with maggot therapy and oral pentoxyfillin. J Dermatol Treat. 2001;12(4):211–4. https://doi.org/10.1080/09546630152696035.
Bender AM, Thompson ED, Hackam DJ, Cameron JL, Rhee DS. Solid pseudopapillary neoplasm of the pancreas in a young pediatric patient: a case report and systematic review of the literature. Pancreas. 2018;47(10):1364–8. https://doi.org/10.1097/MPA.0000000000001183.
Cucchiari D, Torregrosa J-V. Calciphylaxis in patients with chronic kidney disease: a disease which is still bewildering and potentially fatal. Nefrologia. 2018;38(6):579–86. https://doi.org/10.1016/j.nefroe.2018.09.001.
Thosani S, Hu MI. Denosumab: a new agent in the management of hypercalcemia of malignancy. Future Oncol. 2015;11(21):2865–71. https://doi.org/10.2217/fon.15.232.
Helas S, Goettsch C, Schoppet M, et al. Inhibition of receptor activator of NF-κB ligand by denosumab attenuates vascular calcium deposition in mice. Am J Pathol. 2009;175(2):473–8. https://doi.org/10.2353/ajpath.2009.080957.
Samelson EJ, Miller PD, Christiansen C, et al. RANKL inhibition with denosumab does not influence 3-year progression of aortic calcification or incidence of adverse cardiovascular events in postmenopausal women with osteoporosis and high cardiovascular risk. J Bone Miner Res. 2014;29(2):450–7. https://doi.org/10.1002/jbmr.2043.
Raggi P, Bellasi A, Bushinsky D, et al. Slowing progression of cardiovascular calcification with SNF472 in patients on hemodialysis. Circulation. 2020;141(9):728–39. https://doi.org/10.1161/CIRCULATIONAHA.119.044195.
Perelló J, Joubert PH, Ferrer MD, Canals AZ, Sinha S, Salcedo C. First-time-in-human randomized clinical trial in healthy volunteers and haemodialysis patients with SNF472, a novel inhibitor of vascular calcification. Br J Clin Pharmacol. 2018;84(12):2867–76. https://doi.org/10.1111/bcp.13752.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Vijay Kodumudi, George M. Jeha, Nicholas Mydlo and Alan D. Kaye have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kodumudi, V., Jeha, G.M., Mydlo, N. et al. Management of Cutaneous Calciphylaxis. Adv Ther 37, 4797–4807 (2020). https://doi.org/10.1007/s12325-020-01504-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-020-01504-w