Abstract
Microbiota are increasingly studied, providing more precise information on their important role in physiologic processes. They also influence some pathologic processes, such as NSAID-induced enteropathy. This side effect is much more diffuse than it has been described in the past. It derives mainly from the local action of the medicines and is caused by the local binding of gram-negative bacterial lipopolysaccharides and infiltration of neutrophils into the intestinal mucosa. The initial interest in the interaction between these damages and microbiota is very old, but new and interesting data are available. This review aims to focus on recent studies on NSAID-induced enteropathy, an often-underestimated medical condition, and on the influence of microbiota on this condition. Apart from the broadly investigated use of antibiotics and other mucosal protective solutions, this systematic review focuses mostly on the use of probiotics, which directly influence intestinal microflora. Other important factors influencing NSAID-induced enteropathy, such as sex, advanced age, infection and use of proton pump inhibitors, are also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
NSAIDs may be responsible for gastroenteric side effects. Enteritis of this origin is frequently not diagnosed |
Microbiota have a crucial role in the homeostasis of the intestinal mucosa and its functionality |
This systematic review analyzes what has been studied on the interaction between the microbiota and possible NSAID-induced enteritis |
Many studies, both experimental and on humans, have investigated the topic |
Currently, it is clear that the use of probiotics may help in preserving the functionality of the enteral mucosa in patients treated with NSAIDs |
It would be interesting to have clearer data on the the real relationship between the two. These should be based on clinical trials in humans aiming to clarify which kinds of probiotics are most useful to prevent or treat enteritis due to use of any NSAID, especially in patients using them chronically or in combination with low-dose aspirin for cardiovascular risk prevention |
Introduction
Microbiota affect NSAID-induced enteropathy in several ways. They influence the cytotoxicity of bile, enterohepatic circulation of NSAIDs and physiologic process of ulcer healing [1]. The important role of microbiota in NSAID-induced enteropathy was explored many years ago. It was noted that in 3 days oral indomethacin administered to conventional rats produced a syndrome of often fatal of intestinal lesions characterized by multiple ulcers and peritonitis [2]. Germ-free rats were found to be resistant or developed very mild lesions, but they were re-sensitized when exposed to Escherichia coli.
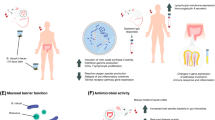
Due to the alarming clinical symptoms of NSAID-induced enteropathy, which include the presence of blood in the stool due to ulceration, anemia of unknown etiology or symptoms of obstruction due to ‘diaphragm-like’ intestinal strictures, many researchers have tried to identify the underlying mechanisms [3]. The initial damage to the mucosal barrier function is attributed to NSAID-induced prostaglandin deficiency and mitochondrial malfunction [3]. The binding of gram-negative bacterial lipopolysaccharides (LPS) as well as the high-mobility group box 1 (HMGB1) from the injured epithelial cells to Toll-like receptor 4 (TLR4) on macrophages leads to the activation of many pathways that release proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and chemokines. This induces neutrophil infiltration into the mucosa and submucosa of the small intestine, causing damage [4] (Fig. 1).
Mechanism underlying the pathophysiology of NSAID-induced intestinal damage. PG prostaglandins, HO-1 heme oxygenase-1, ONOO- peroxynitrite, NO nitric oxide, iNOS inducible nitric oxide synthase, NF-κΒ nuclear factor-κB, NLRP3 pyrin domain-containing 3, TLR4 Toll-like receptor 4, LPS lipopolysaccharide, TNFα tumor necrosis factor α, IL-1β interleukin-1β
In general, an increase in the number of gram-negative bacteria and their LPS in the mucosa induces activation of neutrophils along with the NSAID action and causes ulcer formation [5]. A recent paper has suggested that the correction of the dysbiosis could be an important target to treat intestinal damage after NSAID use and restore normal functionality [6]. It was stated that although it is not always feasible to avoid an induction of dysbiosis, probiotics can be used to correct it. All the mentioned studies attracted our interest and led us to carry out an investigation of what has happened in the last few years in the research on the role of microbiota related to the use of NSAIDs. This systematic review focuses on some aspects of NSAID-induced enteropathy, especially on the use of probiotics to prevent enteric mucosal damage induced by NSAIDs. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Methods
Search
The search was made of electronic databases in December 2019, including PUBMED, Scopus and Cochrane Database of Systematic Reviews. We used a series of logic combinations and research terms related to the topic (“microbiota,” “NSAIDs,” “small intestine,” “gut,” “gastrointestinal tract”) to perform searches in each database. Published systematic reviews on the same topic were reviewed to identify the additional randomized controlled trials. An example of the searching strategy for PUBMED is: (“microbiota” [All Fields]) AND (“Non-Steroidal Anti-Inflammatory Drugs” [All Fields]) AND (“small intestine” [All Fields] OR “gut” OR “gastrointestinal tract” [All Fields]). An additional search was conducted in January 2020 to identify possible studied probiotic supplements against the microbiota dysbiosis. The search words were: (“probiotics” [All Fields] or “probiotic strains” [All Fields] AND “NSAIDS” [All Fields]) AND (“small intestine” [All Fields] or “small bowel” [All Fields] or “large intestine” [All Fields] or “large bowel” [All Fields] or “gastrointestinal tract” [All Fields]). Only papers published in English and whose full text was available were included in this study.
Selection Process
The initial screening of the literature was made examining titles and abstracts and removing duplicates. The eligibility of the studies was double-checked by two independent authors reviewing the full text. Other authors were consulted when uncertainty appeared, such as whether different publications were from the same trial. Disagreements were resolved by discussion between the authors, followed by consulting an external reviewer.
Inclusion and Exclusion Criteria
For the first search, the inclusion criteria were: (1) studies focusing on microbiota changes in the small intestine; (2) the study subjects had received NSAIDs during the study; (3) the studies suggested a mechanism of NSAID action on the small intestine or suggested a treatment/therapeutic approach to lesions in the small intestine. The exclusion criteria were: (1) studies without sufficient data for microbiota; (2) study subjects had received medication other than NSAIDs. The second search was conducted to identify possible therapeutic approaches targeting microbiota alterations in subjects who suffered from NSAIDs enteropathy using the same inclusion and exclusion criteria.
This review also examined the bibliographies from the most recent reviews and additional primary literature sources as well as references cited by relevant articles. All the articles identified were imported to Rayyan. Finally, all included articles were imported to Mendeley as a citation tool.
Objective
Chronic NSAID users are affected to various extents by small intestine injury at a rate of 50–70% [7]. Until recently, no specific therapy had been identified against NSAID-induced enteropathy. Therefore, we conducted this review, integrating a systematic approach to determine any developments regarding the current knowledge. We aim to shed light on possibilities to prevent/cure these lesions using probiotic supplements or enhancing the natural effects of microbiota. This review includes controlled trials in both animals and humans.
Results
The initial search identified 86 publications, while the second identified 53. After duplicates had been removed, we screened 135 articles, excluding 67 of them. Sixty-eight articles were assessed for eligibility to be included in our manuscript; 23 did not meet the inclusions criteria and therefore were excluded. Finally, 45 articles/book chapters were included in the qualitative synthesis (Fig. 2).
Antibiotics and Changes in Microbiota
The changes in gut microbiota after NSAID use were the focus of Terán-Ventura et al. [8] in their controlled trial, where they induced acute indomethacin enteropathy in rats (injecting two doses of the medicine in 48 h) and observed the animals for 4 days. After this period, while the controls showed no histologic changes consistent with the presence of intestinal inflammation, the indomethacin-treated animals showed microscopic signs of inflammation, with significantly worse histopathologic scores. Moreover, although the luminal ileal microbiota of the controls was dominated by Lactobacillus/Enterococcus spp., in the treated group the dominant bacterial groups were Bacteroides spp., Enterobacteriaceae and Clostridium coccoides/Eubacterium rectale, indicative of a state of dysbiosis. The authors concluded that there is a potential role for gut microbiota in the pathophysiology of intestinal inflammation.
Recently, the effect of rifaximin on diclofenac small bowel enteropathy was studied on a rat model [9]. The animals had received diclofenac 4 mg/kg for 2 weeks. A delayed-release formulation of the antibiotic was administered 1 h before the NSAID. Diclofenac induced ileal mucosal lesions through inflammatory pathways and microbiota changes. Rifaximin seemed to prevent diclofenac-induced enteropathy through both antibacterial and antiinflammatory activities. After NSAID administration, TNF, IL-1β, TLR-2, TLR-4, MyD88 and NF-κB levels were increased, while caspase-1 was also activated. Ileal occludin expression (an intestinal epithelial tight junction protein) was decreased, and the balance of bacteria turned with an increase in Proteobacteria and Bacteroidetes. However, rifaximin compensated for all these changes by increasing the proportion of Lactobacilli and also reducing IL-1β production and inhibiting the upstream caspase-1 activation.
Regarding the mechanisms underlying the effects of probiotics on the intestinal mucosa, some recent in vitro studies have shown evidence of the ability of a multistrain probiotic to prevent molecular and cellular damage caused by oxidative and heat-induced stress [10, 11]. Another placebo-controlled study also attributed “eubiotic” properties and antimicrobial activities to rifaximin [12]. Healthy volunteers received diclofenac (75 mg twice daily) plus omeprazole (20 mg once daily) and either rifaximin (400 mg) or placebo twice daily for 14 days. Subjects were assessed by videocapsule endoscopy at baseline and after 2 weeks of treatment. Mucosal breaks were detected in 20% of subjects given rifaximin and in 43% of subjects given placebo, leading the researchers to the above conclusion.
Dealing with the intestinal flora itself, a research group evaluated the changes in mouse gut microbiota following indomethacin administration and observed a significant increase of Firmicutes and decreased quantity of Bacteroidetes [13]. In their trial, the use of antibiotics to reduce gut microbiota led to a higher mortality rate in mice than controls, indicating the crucial role of microbiota. However, mice pre-transplanted with adaptively changed microbiota had less small bowel injury and lower levels of proinflammatory cytokines when exposed to the NSAIDs, showing the importance of bacteria [13]. Moreover, it is suggested that antibiotics targeting gram-negative bacteria are usually effective in reducing the extent of NSAID-induced intestinal ulceration. In contrast, antibiotics against gram-positive bacteria do not appear to play a significant role [14]. Other important information regarding antibiotics and NSAIDs is the dynamic interplay between the two medication groups. One study showed that a single oral dose of indomethacin elicited changes in the composition and diversity of the microbiota. Moreover, the microbiota changed indomethacin’s pharmacokinetics and pharmacodynamics as well as its effectiveness [15].
Type of NSAID and Microbiota Composition Difference
Although many studies consider NSAIDs as a single category of medication, when it comes to microbiota changes, significant differences seem to take place. In aspirin-treated patients, Prevotella spp., Bacteroides spp., family Ruminococaceae and Barnesiella spp. were identified. In contrast, in the celecoxib/ibuprofen users there was an abundance of Acidaminococcaceae and Enterobacteriaceae. Moreover, bacteria from the families Propionibacteriaceae, Pseudomonadaceae, Puniceicoccaceae and Rikenellaceae were most common in people on ibuprofen rather than naproxen [16]. Finally, in the same study, Bacteroides spp. and Erysipelotrichaceae spp. discriminated between subjects using NSAIDs with proton pump inhibitors (PPIs) and those using NSAIDs alone. The role of PPIs will be more extensively discussed in this article.
A very important pilot study showed amelioration of small bowel injury by switching from non-selective NSAIDs to celecoxib in patients with rheumatoid arthritis [17]. Conversion to COX-2 selective NSAIDs for 12 weeks significantly reduced the number of petechiae and red spots in the gut, the number of mucosal breaks and Lewis scores in endoscopic images.
A former study investigated two COX-2 selective NSAIDs, celecoxib and etoricoxib, along with two nonselective NSAIDs, indomethacin and diclofenac. The latter reduced hemoglobin levels, whereas etoricoxib and celecoxib did not have any effects. Moreover, celecoxib caused less damage compared with the other NSAIDs. Their findings suggest that nonselective NSAIDs and etoricoxib can induce enteropathy through a topical action, whereas celecoxib lacks relevant detrimental actions [18].
High Fat Diet and Dysbiosis
As lower intestinal damage comprises an imminent danger, many scientists have tried to come up with protective solutions against inflammation and dysbiosis. Sugimura et al. [19] showed that a high fat diet could induce small intestinal damage through gut dysbiosis in NSAID-treated animals. The mice fed this type of diet for 2 months ended up with significantly decreased Bifidobacterium spp. populations. Moreover, the intestinal permeability was increased, while there was a ptosis in protein expressions of zonula occludens-1 (ZO-1) and occludin, which are both tight junction proteins. Interleukin (IL)-17A levels in the small intestine were also higher than expected. In this study, normal diet-fed mice received small intestinal microbiota from high-fat diet-fed mice and had the same symptoms and findings. Neutralizing antibodies against IL-17A were given to the transplanted mice, and the symptoms were significantly reduced as a consequence. The researchers concluded that HFD-induced alterations of small intestinal microbiota cause microinflammation through the induction of IL-17A, suggesting a therapeutic strategy against this factor. Moreover, an increase in intestinal permeability was noted, which aggravated NSAID-induced small intestinal damage. Their suggestions were that low-fat dietary therapy and strategies to enhance individual-specific innate functions of Bifidobacterium may be useful for the prevention and mitigation of NSAID-induced small intestinal damage.
Influence of probiotics on NSAID-induced enteropathy
Bifidobacterium breve
Recently, a randomized, double-blind trial of healthy volunteers who received 300 mg acetylsalicylic acid (ASA) daily for 6 weeks found that the concomitant use of Bifidobacterium breve (Bif195, 5 × 1010 colony-forming units) safely reduced the risk of small-intestinal enteropathy caused by ASA [20].
Lactobacillus gasseri (LG) OLL2716
Another group has identified Lactobacillus gasseri (LG) OLL2716 as possibly useful in reducing aspirin-induced small bowel injuries and in mitigating gastrointestinal symptoms [21]. In this double-blind study, patients received aspirin for > 1 month and 112 ml yogurt containing LG or placebo twice daily for 6 weeks. Before and after treatment, small bowel injuries were evaluated by capsule endoscopy, and symptoms were assessed using the Frequency Scale for the Symptoms of Gastroesophageal Reflux Disease (FSSG) and the Gastrointestinal Symptom Rating Scale (GSRS) questionnaires. According to the findings, the LG group had significantly fewer small bowel mucosal breaks and reddened lesions, while the FSSG and GSRS scores were also significantly improved.
Lactobacillus plantarum TIFN101 and WCFS1
Three Lactobacillus plantarum strains (L. plantarum WCFS1, CIP104448, TIFN101) were investigated for their effects on in-vivo small intestinal barrier function and gut mucosal gene transcription [22]. The selection of these specific strains was made through their differential effects on Toll-like receptor signaling and tight junction protein rearrangement. In this randomized, double-blind placebo-controlled cross-over trial, the patients received indomethacin and then sequentially the different strains with a washout period. None of the strains had an effect on the lactulose-rhamnose ratio (an indicator of small intestinal permeability), which increased after administration of indomethacin. However, L. plantarum TIFN101 proved to have more repairing properties. L. plantarum TIFN101 was also found to prevent the serum reduction of CD4+/Foxp3 regulatory cells, caused by NSAIDs, by enhancing the responses against tetanus toxoid (TT) antigen. It was also found to upregulate genes associated with the maintenance of T- and B-cell function and antigen presentation. Finally, L. plantarum TIFN101 and WCFS1 were both able to downregulate immunologic pathways involved in antigen presentation, showing indications of tissue repair [23].
High-concentration multi-strain probiotic mixture
The probiotic mixture VSL#3 has been extensively investigated and is currently recommended for the prevention and treatment of chronic pouchitis and ulcerative colitis [24,25,26]. A study describes the effects of VSL#3, containing 450 billion freeze-dried bacteria (Streptococcus thermophilus, Bifidobacterium longum, B. breve, B. infantis, Lactobacillus acidophilus, L. plantarum, L. casei, L. Bulgaricus), administered for 3 weeks, on the survival rates and small intestinal injuries in indomethacin-treated subjects [27]. The improvements appeared to be closely associated with a decrease in proinflammatory cytokines such as IL-1β, IL-6 and TNF-α as well as an increase in the antiinflammatory cytokine IL-10 [27, 28].
Bifidobacterium adolescentis
This was found to significantly reduce naproxen-induced small intestinal enteropathy in rats, which was attributed to its ability to produce high levels of lactic acid [29]. Rats were allocated to three different groups, where for 5 days they were administered a prebiotic inulin, or a vehicle, or a high lactate-producing Bifidobacterium adolescentis (109 CFU) or a low lactate-producing Bifidobacterium longum strain (109 CFU). After that, naproxen small intestinal enteropathy was induced, and the damage was blindly scored at the end of the treatment period. Their results showed that intestinal damage was similar in rats treated with inulin or vehicle, while treatment with B. adolescentis resulted in an 82% reduction in the extent of intestinal damage. Additionally, B. longum did not show any beneficial effect. Therefore, it was suggested that the high lactate-producing Bifidobacterium-based probiotics might represent a viable approach to NSAID-induced enteropathy.
A recent publication examined the protective effects of a combination of the probiotic Bifidobacterium longum BB536 (2.5 × 106 CFU/rat twice daily) with the prebiotic lactoferrin (100 mg/kg twice daily) in a rat model of diclofenac-induced enteropathy [30].
Lactobacillus casei Shirota
Lactobacillus casei Shirota exhibits a prophylactic effect on indomethacin-induced enteropathy in rats by suppressing the lipoproteins/Toll-like receptor 4 (LPS/TLR4) signaling pathway [31]. This effect may be mediated by l-lactic acid.
Other Treatments Affecting Microbiota
Rebapamide
Rebapamide, a mucosal protective drug, apart from its inflammation-suppressing ability, was found to inhibit indomethacin-induced small intestinal mucosal damage by modulating the microbiota [32]. The number of Enterococcaceae and Enterobacteriaceae in the jejunal mucosa were restored to normal levels, and the segmented filamentous bacteria were increased.
Diallyl disulfide (DADS)
The ability of a hydrogen sulfide donor, diallyl disulfide, an important mediator of gastrointestinal mucosal defense, to protect against NSAID-induced enteropathy has been investigated [1]. When rats were treated with a protective dose of DADS (30 mmol/kg), multiple Clostridiales families were significantly decreased, such as Ruminococcaceae and Eubacteriaceae; Enterococcaceae were decreased, and the Mucispirillum family was increased. Thus, the above changes in the microbiota induced by administration of diallyl disulfide may have contributed significantly to the severity of naproxen-induced intestinal damage.
Other Information Regarding NSAID Enteropathy (Stress, Age, CDI, PPIS)
Stress conditions
Stressful situations in mice were shown to exacerbate NSAID-induced small bowel injury by inducing changes in intestinal microbiota [33]. Also, it was found to increase both the total number of bacteria and the proportion of gram-negative bacteria as well as the permeability of enteral mucosa via glucocorticoid receptor signaling.
Sex differences
As sex is a variable for multiple health conditions, a clinical study investigated the differences in microbiota and NSAID enteropathy in healthy men and women studying biopsy samples [34]. An important finding was that the composition of intestinal flora did not differ between sexes. However, one further observation was that after indomethacin administration, women had lower intestinal permeability and higher microbial diversity than men. These changes were normalized after discontinuation of the medication. Moreover, only women demonstrated decreased fecal microbial diversity after indomethacin, including an increase in Prevotella abundance.
Age
A very interesting study reported the impact of age on microbial communities in people using NSAIDs [35]. The total number of microbes in elderly NSAID users was higher than in the elderly without NSAIDs as shifts in all major microbial phyla, such as lower numbers of Firmicutes and an increase in numbers of Bacteroidetes, were observed. Moreover, in the elderly taking NSAIDs, there were reductions in the proportion of butyrate producers belonging to Clostridium cluster XIVa, such as Roseburia and Ruminococcus. Additionally, regarding the Actinobacteria group, lower numbers of Collinsella spp. were identified in contrast to young adults or elderly not using NSAIDs. The above served as an indication that NSAID usage along with advanced age could influence the composition of intestinal microbiota. Finally, relatively high numbers of Lactobacillus appeared only in the NSAID-free elderly patients.
Clostridium difficile infection
Important information regarding NSAID use and the disturbance of microbiota was obtained during Clostridium difficile infection (CDI) [36]. The use of NSAIDs has been associated with enhanced susceptibility and severity of CDI. In mice, the experimental use of indomethacin before infection dramatically increased mortality and intestinal pathology. Alterations in microbiota seem to play an important role in the underlying mechanisms.
Proton Pump Inhibitors
Wallace et al. [37] investigated the effect of the simultaneous consumption of NSAIDs and proton pump inhibitors (PPIs) on the gut microbiota. After administering antisecretory doses of omeprazole or lansoprazole for 9 days and antiinflammatory doses of naproxen or celecoxib for 4 days to rats, they evaluated enteropathy and measured possible microhemorrhages. They concluded that both PPIs significantly worsen selective and non-selective NSAID enteropathy. A very important observation was that omeprazole treatment significantly reduced jejunal Actinobacteria and Bifidobacteria spp. (around 80%). In contrast, supplementation of selected commensal bacteria (Bifidobacteria enriched) during treatment with omeprazole and naproxen prevented intestinal ulceration and bleeding. Moreover, the severity of enteropathy increased when implanting germ-free mice with jejunal bacteria from PPI-treated rats. Physicians should carefully consider the crucial importance of possible intestinal damage and bleeding in their high-risk patients before prescribing PPIs and NSAIDs as well as low-dose aspirin concomitantly. It is essential to be aware of the new knowledge regarding this coadministration. Although PPIs and NSAIDs have been investigated in many studies in the past, the exact mechanisms of their interaction have not been clearly identified. Recently, a group of researchers compared PPIs with vonoprazan fumarate, a gastric acid secretion inhibitor with a different mechanism of action, to identify whether the PPI drug class induces dysbiosis or this depends on the inhibition of gastric acid itself [38]. They concluded that the cause of deterioration of NSAID-induced enteropathy is the decreased population of L. johnsonii in the small intestine, as the administration of these bacteria ameliorated NSAID-induced small intestinal injury at doses ≥ 106. In contrast, a dose of 105 did not alter the injury. Moreover, they found that L. murinus but not L. intestinalis ameliorated indomethacin-induced minor intestinal injury. This group rejected Wallace’s proposal to treat NSAID-induced minor intestinal injury associated with PPI treatment by administering Actinobacteria. They also found a reduction in the population of Actinobacteria in the small intestine after rabeprazole and vonoprazan, but they stated that this reduction is negligible compared with the decrease in L. johnsonii.
Conclusions
This review has highlighted the latest information from the literature regarding the often underestimated NSAID enteropathy. Specifically, it has analyzed the most recent research regarding the possible administration of probiotics to restore the intestinal microbiota and reduce NSAID-induced enteric damages. Until now, the administration of selected probiotic strains seems very promising. However, it is crucial that investigation continues, and the next steps with larger human randomized clinical trials are made. This would definitely provide more important scientific material to discuss. It is important to highlight that trials including human patients with chronic NSAID use have been conducted rather than clinical trials with a short-term high dosage of NSAIDs. The latter might reveal many differences in the microbiota of chronic NSAID users. In addition to the patient’s sex and age, consistent with the growing need to provide tailored therapies, clinicians should also take into account the broad diversity of the gut microbiota and the individual susceptibility to NSAIDs, especially in the presence of intestinal infections such as C. difficile, and the contextual intake of other drugs such as PPIs.
Finally, as strongly suggested by an increasing number of studies [39,40,41,42,43,44,45], a careful selection of the probiotic agent, standardization of the dose and detailed characterization of the beneficial effects are essential when considering use of a probiotic for the management of NSAID-induced enteropathy. Indeed, the bioavailability of nutrients, drugs, probiotics or other substances administered orally depends on intestinal barrier integrity, which can be compromised in various inflammatory gut diseases, including NSAID-induced enteropathy.
References
Blackler RW, Motta JP, Manko A, Workentine M, Bercik P, Surette MG, et al. Hydrogen sulphide protects against NSAID-enteropathy through modulation of bile and the microbiota. Br J Pharmacol. 2015;172(4):992–1004. https://doi.org/10.1111/bph.12961.
Robert A, Asano T. Resistance of germfree rats to indomethacin-induced intestinal lesions. Prostaglandins. 1977;14(2):333–41. https://doi.org/10.1016/0090-6980(77)90178-2.
Montalto M, Gallo A, Gasbarrini A, Landolfi R. NSAID enteropathy: could probiotics prevent it? J Gastroenterol. 2013;48(6):689–97. https://doi.org/10.1007/s00535-012-0648-2.
Otani K, Tanigawa T, Watanabe T, Shimada S, Nadatani Y, Nagami Y, et al. Microbiota plays a key role in non-steroidal anti-inflammatory drug-induced small intestinal damage. Digestion. 2017;95(1):22–8. https://doi.org/10.1159/000452356.
Blackler RW, De Palma G, Manko A, Da Silva GJ, Flannigan KL, Bercik P, Surette MG, Buret AG, Wallace JL. Deciphering the pathogenesis of NSAID enteropathy using proton pump inhibitors and a hydrogen sulfide-releasing NSAID. Am J Physiol Gastrointest Liver Physiol. 2015;308:994–1003. https://doi.org/10.1152/ajpgi.00066.2015.
Syer SD, Wallace JL. Environmental and NSAID-enteropathy: dysbiosis as a common factor. Curr Gastroenterol Rep. 2014;16(3):377. https://doi.org/10.1007/s11894-014-0377-1.
Greenson JK. Diagnostic Pathology: Gastrointestinal E-Book. Amsterdam: Elsevier Health Sciences; 2015.
Terán-Ventura E, Aguilera M, Vergara P, Martínez V. Specific changes of gut commensal microbiota and TLRs during indomethacin-induced acute intestinal inflammation in rats. J Crohns Colitis. 2014;8(9):1043–54.
Colucci R, Pellegrini C, Fornai M, Tirotta E, Antonioli L, Renzulli C, Ghelardi E, Piccoli E, Gentile D, Benvenuti L, Natale G, Fulceri F, Palzon-Riquelme P, Lopez-castejon G, Blandizzi C, Scarpiganto C. Pathophysiology of NSAID-associated intestinal lesions in the rat: luminal bacteria and mucosal inflammation as targets for prevention. Front Pharmacol. 2018;9:1340. https://doi.org/10.3389/fphar.2018.01340.
Cinque B, La Torre C, Lombardi F, Palumbo P, Evtoski Z Jr, Santini S, Falone S, Cimini A, Amicarelli F, Cifone MG. VSL#3 probiotic differently influences IEC-6 intestinal epithelial cell status and function. J Cell Physiol. 2017;232(12):3530–9. https://doi.org/10.1002/jcp.25814.
Palumbo P, Lombardi F, Cifone MG, Cinque B. The epithelial barrier model shows that the properties of VSL#3 depend from where it is manufactured. Endocr Metab Immune Disord Drug Targets. 2019;19(2):199–206. https://doi.org/10.2174/1871530318666181022164505.
Scarpignato C, Dolak W, Lanas A, Matzneller P, Renzulli C, Grimaldi M, Zeitlinger M, Bjarnason I. Rifaximin reduces the number and severity of intestinal lesions associated with use of nonsteroidal anti-inflammatory drugs in humans. Gastroenterology. 2017;152(5):980–982.e3. https://doi.org/10.1053/j.gastro.2016.12.007.
Xiao X, Nakatsu G, Jin Y, Wong S, Yu J, Lau JYW. Gut microbiota mediates protection against enteropathy induced by indomethacin. Sci Rep. 2017;7:40317. https://doi.org/10.1038/srep40317.
Syer SD, Blackler RW, Martin R, de Palma G, Rossi L, Verdu E, Bercik P, Surette MG, Aucouturier A, Langella P, Wallace JL. NSAID enteropathy and bacteria: a complicated relationship. J Gastroenterol. 2015;50(4):387–93. https://doi.org/10.1007/s00535-014-1032-1.
Liang X, Bittinger K, Li X, Abernethy DR, Bushman FD, FitzGerald GA. Bidirectional interactions between indomethacin and the murine intestinal microbiota. Elife. 2015;4:e08973. https://doi.org/10.7554/eLife.08973.
Rogers MAM, Aronoff DM. The influence of non-steroidal anti-inflammatory drugs on the gut microbiome. Clin Microbiol Infect. 2016;22(2):178.e1–.e9. https://doi.org/10.1016/j.cmi.2015.10.003.
Inoue T, Iijima H, Arimitsu J, Hagihara K, Kawai S, Shiraishi E, et al. Amelioration of small bowel injury by switching from nonselective nonsteroidal anti-inflammatory drugs to celecoxib in rheumatoid arthritis patients: a pilot study. Digestion. 2014;89(2):124–32. https://doi.org/10.1159/000357229.
Fornai M, Antonioli L, Colucci R, Pellegrini C, Giustarini G, Testai L, et al. NSAID-induced enteropathy: are the currently available selective COX-2 inhibitors all the same? J Pharmacol Experim Therap. 2014;348(1):86–95.
Sugimura N, Otani K, Watanabe T, Nakatsu G, Shimada S, Fujimoto K, et al. High-fat diet-mediated dysbiosis exacerbates NSAID-induced small intestinal damage through the induction of interleukin-17A. Sci Rep. 2019;9(1):16796. https://doi.org/10.1038/s41598-019-52980-2.
Mortensen B, Murphy C, O’Grady J, Lucey M, Elsafi G, Barry L, et al. Bifidobacteriumbreve Bif195 protects against small-intestinal damage caused by acetylsalicylic acid in healthy volunteers. Gastroenterology. 2019;157(3):637–646.e4. https://doi.org/10.1053/j.gastro.2019.05.008.
Suzuki T, Masui A, Nakamura J, Shiozawa H, Aoki J, Nakae H, et al. Yogurt containing lactobacillus gasseri mitigates aspirin-induced small bowel injuries: a prospective, randomized, double-blind, placebo-controlled trial. Digestion. 2017;95(1):49–544. https://doi.org/10.1159/000452361.
Mujagic Z, de Vos P, Boekschoten MV, Govers C, Pieters HJHM, de Wit NJW, et al. The effects of Lactobacillus plantarum on small intestinal barrier function and mucosal gene transcription: a randomized double-blind placebo controlled trial. Sci Rep. 2017;7:40128. https://doi.org/10.1038/srep40128.
de Vos P, Mujagic Z, de Haan BJ, Siezen RJ, Bron PA, Meijerink M, et al. Lactobacillus plantarum strains can enhance human mucosal and systemic immunity and prevent non-steroidal anti-inflammatory drug induced reduction in T regulatory cells. Front Immunol. 2017;8:1000. https://doi.org/10.3389/fimmu.
Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, Poggioli G, Miglioli M, Campieri M. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119(2):305–9.
Ulisse S, Gionchetti P, D’Alo S, Russo FP, Pesce I, Ricci G, Rizzello F, Helwig U, Cifone MG, Campieri M, De Simone C. Expression of cytokines, inducible nitric oxide synthase, and matrix metalloproteinases in pouchitis: effects of probiotic treatment. Am J Gastroenterol. 2001;96(9):2691–9. https://doi.org/10.1111/j.1572-0241.2001.04139.x.
Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, Brigidi P, Vitali B, Poggioli G, Miglioli M, Campieri M. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124(5):1202–9. https://doi.org/10.1016/s0016-5085(03)00171-9.
Montalto M, Gallo A, Curigliano V, D’Onofrio F, Santoro L, Covino M, et al. Clinical trial: the effects of a probiotic mixture on non-steroidal anti-inflammatory drug enteropathy—a randomized, double-blind, cross-over, placebo-controlled study. Aliment Pharmacol Ther. 2010;32(2):209–14. https://doi.org/10.1111/j.1365-2036.2010.04324.x.
Soo I, Madsen KL, Tejpar Q, Sydora BC, Sherbaniuk R, Cinque B, Di Marzio L, Cifone MG, De Simone C, Fedorak RN. VSL#3 probiotic upregulates intestinal mucosal alkaline sphingomyelinase and reduces inflammation. Can J Gastroenterol. 2008;22(3):237–42. https://doi.org/10.1155/2008/520383.
Syer SD, McKnight W, Aucouturier A, Martin R, Langella P, Wallace JL. Su1724 Bifidobacteria exert a protective effect against NSAID-induced enteropathy that is dependent on lactate production. Gastroenterology. 2012;142(5):S-489.
Fornai M, Pellegrini C, Benvenuti L, Tirotta E, Gentile D, Natale G, Ryskalin L, Colucci R, Piccoli E, Ghelardi E, Blandizzi C, Antonioli L. Protective effects of the combination bifidobacterium longum plus Lactoferrin against NSAID—induced enteropathy. Nutrition. 2020;70:110583. https://doi.org/10.1016/j.nut.2019.110583.
Watanabe T, Nishio H, Tanigawa T, Yamagami H, Okazaki H, Watanabe K, et al. Probiotic Lactobacillus casei strain Shirota prevents indomethacin-induced small intestinal injury: involvement of lactic acid. Am J Physiol Gastrointest Liver Physiol. 2009;297:G506–513. https://doi.org/10.1152/ajpgi.90553.2008.
Kurata S, Nakashima T, Osaki T, Uematsu N, Shibamori M, Sakurai K, et al. Rebamipide protects small intestinal mucosal injuries caused by indomethacin by modulating intestinal microbiota and the gene expression in intestinal mucosa in a rat model. J Clin Biochem Nutr. 2015;56(1):20–7. https://doi.org/10.3164/jcbn.14-67.
Yoshikawa K, Kurihara C, Furuhashi H, Takajo T, Maruta K, Yasutake Y, et al. Psychological stress exacerbates NSAID-induced small bowel injury by inducing changes in intestinal microbiota and permeability via glucocorticoid receptor signaling. J Gastroenterol. 2017;52(1):61–71. https://doi.org/10.1007/s00535-016-1205-1.
Edogawa S, Peters SA, Jenkins GD, Gurunathan SV, Sundt WJ, Johnson S, et al. Sex differences in NSAID-induced perturbation of human intestinal barrier function and microbiota. FASEB J. 2018;32(12):6615–25. https://doi.org/10.1096/fj.201800560R.
Mäkivuokko H, Tiihonen K, Tynkkynen S, Paulin L, Rautonen N. The effect of age and non-steroidal anti-inflammatory drugs on human intestinal microbiota composition. Br J Nutr. 2010;103(2):227–34. https://doi.org/10.1017/S0007114509991553.
Zackular JP, Kirk L, Trindade BC, Skaar EP, Aronoff DM. Misoprostol protects mice against severe Clostridium difficile infection and promotes recovery of the gut microbiota after antibiotic perturbation. Anaerobe. 2019;58:89–94. https://doi.org/10.1016/j.anaerobe.2019.06.006.
Wallace JL, Syer S, Denou E, de Palma G, Vong L, McKnight W, et al. Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology. 2011;141(4):1314–22. https://doi.org/10.1053/j.gastro.2011.06.075.
Nadatani Y, Watanabe T, Suda W, Nakata A, Matsumoto Y, Kosaka S, et al. Gastric acid inhibitor aggravates indomethacin-induced small intestinal injury via reducing Lactobacillus johnsonii. Sci Rep. 2019;9(1):17490. https://doi.org/10.1038/s41598-019-53559-7.
Biagioli M, Laghi L, Carino A, Cipriani S, Distrutti E, Marchiano S, Parolin C, Scarpelli P, Vitali B, Fiorucci S. Metabolic variability of a multispecies probiotic preparation impacts on the anti-inflammatory activity. Front Pharmacol. 2017;8:505. https://doi.org/10.3389/fphar.2017.00505.
Cinque B, La Torre C, Lombardi F, Palumbo P, Evtoski Z, Santini S Jr, Falone S, Cimini A, Amicarelli F, Cifone MG. VSL#3 probiotic di_erently influences IEC-6 intestinal epithelial cell status and function. J Cell Physiol. 2017;232:3530–9. https://doi.org/10.1002/jcp.25814.
Cinque B, La Torre C, Lombardi F, Palumbo P, Van der Rest M, Cifone MG. Production conditions affect the in vitro anti-tumoral effects of a high concentration multi-strain probiotic preparation. PLoS ONE. 2016;11:e0163216. https://doi.org/10.1371/journal.pone.0163216.
Palumbo P, Lombardi F, Cifone MG, Cinque B. The epithelial barrier model shows that the properties of VSL#3 depend from where it is manufactured. Endocr Metab Immune Disord Drug Targets. 2019;19:199–206. https://doi.org/10.2174/1871530318666181022164505.
Sanders ME, Klaenhammer TR, Ouwehand AC, Pot B, Johansen E, Heimbach JT, Marco ML, Tennila J, Ross RP, Franz C, et al. Effects of genetic, processing, or product formulation changes on efficacy and safety of probiotics. Ann N Y Acad Sci. 2014;1309:1–18. https://doi.org/10.1111/nyas.12363.
Trinchieri V, Laghi L, Vitali B, Parolin C, Giusti I, Capobianco D, Mastromarino P, De Simone C. Efficacy and safety of a multistrain probiotic formulation depends from manufacturing. Front Immunol. 2017;8:1474. https://doi.org/10.3389/fimmu.2017.01474.
Zacarias MF, Souza TC, Zaburlin N, Carmona Cara D, Reinheimer J, Nicoli J, Vinderola G. Influence of technological treatments on the functionality of bifidobacterium lactis INL1, a breast milk-derived probiotic. J Food Sci. 2017;82:2462–70. https://doi.org/10.1111/1750-3841.
Acknowledgements
The authors thank Mariella Fusco for her reviewer support in the selection of papers. They are also grateful to Luca Di Giacomo for the assistance with the graphic design.
Funding
This research has been made possible thanks to an unconditional grant provided by the “Paolo Procacci Foundation-ONLUS,” Via Tacito 7, 00193 Rome, Italy. It was also partially funded by a grant received from “Fondazione Internazionale Salvatore Maugeri,” research project “Modello di Unità di Terapia del Dolore e di Cure Palliative, integrate con Associazioni di Volontariato.” No Rapid Service Fee was received by the journal for the publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
M. Rekatsina carried out most of the research and drafted the initial manuscript. All the authors have contributed to reviewing and ameliorating the quality of the paper and have reviewed and approved the final draft of the manuscript.
Disclosures
Martina Rekatsina, Maria Grazia Cifone and Francesca Lombardi have nothing to disclose. Antonella Paladini, Joseph V. Pergolizzi and Giustino Varrassi are members of the journal’s Editorial Board.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by the authors without a previous Ethics Committee approval.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.12063216.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Rekatsina, M., Paladini, A., Cifone, M.G. et al. Influence of Microbiota on NSAID Enteropathy: A Systematic Review of Current Knowledge and the Role of Probiotics. Adv Ther 37, 1933–1945 (2020). https://doi.org/10.1007/s12325-020-01338-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-020-01338-6