Abstract
Introduction
Occlusive portal venous system thrombosis (PVT) is significantly associated with poor outcomes in cirrhotic patients. Nonselective β-blockers (NSBBs) may be associated with the development of PVT. However, the role of NSBBs in progressing thrombosis remains unclear.
Methods
Forty-three patients on whom contrast-enhanced computed tomography or magnetic resonance imaging was performed twice, and for whom there was detailed information regarding NSBBs, were eligible in this study, including 16 in the NSBBs group and 27 in the no NSBBs group. A composite endpoint of progressing thrombosis included the development of PVT in patients without PVT and aggravation of PVT in patients with PVT. Logistic regression analysis was employed to identify the effect of NSBBs on the progression of PVT.
Results
At the last admission, 13 patients had progressing thrombosis. The incidence of progressing thrombosis was significantly higher in the NSBBs group than in the no NSBBs group [50.0% (8/16) vs. 18.5% (5/27), P = 0.030]. The use of NSBBs (odds ratio 4.400, 95% confidence interval 1.107–17.482, P = 0.035) was significantly associated with progressing thrombosis in univariate logistic regression analyses, but not significant (odds ratio 4.084, 95% confidence interval 0.488–34.158, P = 0.194) in multivariate logistic regression analyses.
Conclusions
NSBBs may play a role in the progression of PVT in liver cirrhosis. The benefits and risks of NSBBs in the management of liver cirrhosis should be fully weighed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Nonselective β blockers (NSBBs), which are recommended for the primary and secondary prophylaxis of esophageal variceal bleeding in cirrhotic patients, may be associated with the development of portal venous system thrombosis (PVT). |
The role of long-term use of NSBBs in progressing thrombosis remains unclear. |
What was learned from the study? |
There was a higher incidence of progressing thrombosis in cirrhotic patients who received NSBBs than in those who did not receive NSBBs. |
NSBBs might facilitate the progression of PVT in cirrhosis. The benefits and risks of NSBBs in the management of liver cirrhosis should be fully weighed. |
Introduction
Liver cirrhosis often leads to serious complications [1], such as liver failure [2], hepatic encephalopathy [3], ascites [4], and spontaneous portosystemic shunts [5]. Recent evidence suggests that portal venous system thrombosis (PVT) is also significantly associated with poor outcomes, with increased morbidity and mortality in cirrhotic patients [6, 7]. The yearly incidence of PVT is from 2.4 to 17.9% [7,8,9]. Decreased portal vein velocity has been identified as a risk factor for the development of PVT [9,10,11]. Nonselective β-blockers (NSBBs), including propranolol and nadolol, which are generally recommended for the primary and secondary prophylaxis of esophageal variceal bleeding in cirrhotic patients [12,13,14], can significantly decrease the portal vein velocity [15,16,17]. Thus, we hypothesized that NSBBs might induce the development of PVT [18], and our recent meta-analysis confirmed this association [19]. However, due to its benefits in decreasing the risk of bleeding and death, the long-term use of NSBBs remains necessary.
The current study aimed to further elucidate the role of NSBBs in thrombus aggravation among cirrhotic patients with PVT.
Methods
Study Design
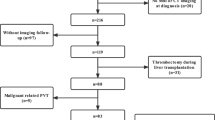
Based on a prospectively collected database in which eligible patients should be diagnosed with liver cirrhosis without malignancy and performed both contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) and upper gastrointestinal endoscopy during their hospitalizations, we retrospectively screened the patients admitted between August 2013 and October 2019 and selected the patients who performed contrast-enhanced CT and/or MRI scans again to assess the progression of liver disease at their repeated admission. The exclusion criteria were as follows: (1) patients with a history of liver transplantation, splenectomy, or other abdominal trauma; (2) patients received anticoagulants or antiplatelets during study period; (3) contrast-enhanced CT and/or MRI scans were not well preserved; (4) the information regarding use of NSBBs was unavailable; and (5) the duration between baseline and follow-up contrast-enhanced CT/MRI scans was less than 6 months.
The study was conducted in accordance with the Helsinki Declaration and approved by the Medical Ethical Committee of the General Hospital of Northern Theater Command. Due to the retrospective observational nature of the study, the requirement for patient consent was waived.
Data Collection
Age, gender, etiology of liver cirrhosis, laboratory tests at the first admission, including hemoglobin, platelet count, total bilirubin, serum albumin, alanine aminotransferase, aspartate aminotransferase, serum creatinine, sodium, and international normalized ratio at the last admission were collected for all patients; Child–Pugh score, which is composed of ascites, hepatic encephalopathy, total bilirubin, serum albumin, and international normalized ratio, was calculated; and model for end-stage liver disease (MELD) score, which is composed of total bilirubin, serum creatinine, and international normalized ratio, was also calculated [20]. The type, dosage, frequency, and duration of NSBBs were collected. The presence and severity of PVT at the first and last admissions were evaluated by three physicians (FY, SX, and XQ), who were blinded to the use of NSBBs.
NSBBs
Generally, the dosage and frequency of NSBBs followed the recommendations of current guidelines [12,13,14]. The detailed information regarding the type, dosage, duration, and adherence of NSBBs was acquired by telephone follow-up. Patients were divided into NSBBs and no NSBBs groups according to the use of NSBBs during the study period. The reasons why these patients did not take NSBB included: (1) contraindication or intolerance to NSBB, and (2) the use of NSBB was not given at the discretion of their physicians. Notably, only patients who had taken NSBBs for 6 months or more were classified as the NSBBs group; in contrast, patients who had never taken NSBBs or had taken NSBBs for less than 6 months were classified as the no NSBBs group.
PVT
Contrast-enhanced CT and/or MRI scans were used to determine the presence of PVT. The degree of PVT was recorded based on the most severe thrombosis in any vessel of the portal venous system, including mural PVT (< 50% occlusion), partial PVT (≥ 50% occlusion), complete PVT (> 80% occlusion), and fibrotic cord. The extension of PVT was recorded as follows, including left portal vein (LPV), right portal vein (RPV), main portal vein (MPV), confluence of superior mesenteric vein (SMV) and splenic vein (SV), SV, and SMV.
Progressing thrombosis was a composite endpoint, which included the development and aggravation of PVT (Fig. 1). The dynamic change of PVT was obtained by comparing the findings of contrast-enhanced CT/MRI scans between baseline and follow-up. The development of PVT was defined as de novo occurrence of a thrombus within the portal venous system in patients without PVT. The aggravation of PVT was defined as either the degree or the extension of the thrombus was aggravated in patients with PVT. To standardize this definition of PVT aggravation, a quantitative scoring system was employed. First, according to the degree of the thrombus at each vessel of the portal venous system, mural thrombus (< 50% occlusion), partial thrombus (≥ 50% occlusion), complete thrombus (> 80% occlusion), and fibrotic cord were assigned to 1, 2, 3, and 4 points, respectively. Then, an accumulative score was calculated by adding the points for each individual patient. Finally, the scores obtained between the last and first admissions were subtracted to evaluate a dynamic change of severity of PVT. A positive value (> 0) indicated the aggravation of PVT; otherwise, the aggravation of PVT would not be considered.
Contrast-enhanced computed tomography (CT) scans in a patient with progression of portal venous system thrombosis (PVT) after a continuous use of propranolol for 1.77 years. Upper panels referred to contrast-enhanced CT images on November 2017, which showed no thrombosis in the left portal vein (LPV) (score = 0), mural thrombus in the right portal vein (RPV) (score = 1), mural thrombus in the main portal vein (MPV) (score = 1), mural thrombus at the confluence of the superior mesenteric vein (SMV) and splenic vein (SV) (score = 1), SV (score = 2), and SMV (score = 0). An accumulative score was 5 points at the first admission. Lower panels refer to contrast-enhanced CT images on October 2019, which showed no thrombosis in LPV (score = 0), mural thrombus in RPV (score = 1), mural thrombus in MPV (score = 1), complete thrombus in confluence of SMV and SV (score = 3), SV (score = 2), and SMV (score = 0). An accumulative score was 7 points at the last admission
Statistical Analysis
Statistical analysis was performed by using the SPSS 20.0 (IBM, College Station, USA) statistical package. Continuous variables were expressed as median (range) and categorical variables as frequency (percentage). Differences in the continuous variables were evaluated by the Mann–Whitney U test and categorical variables were evaluated by the Chi square test. Univariate and multivariate logistic regression analyses were performed to identify the effect of NSBBs on progressing thrombosis in liver cirrhosis. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. A P value < 0.05 was considered to be statistically significant.
Results
Patients
A total of 43 patients were included in this study, including 16 in the NSBBs group and 27 in the no NSBBs group (Fig. 2). Patient characteristics are summarized in Table 1. The median age was 57 years (range 30–76 years) and 35 (81.4%) patients were male. The most common etiology of cirrhosis was alcohol abuse (22, 51.2%) and hepatitis B viral infection (15, 34.9%). A majority of patients had Child–Pugh class A (22, 52.4%). Median MELD score at admission was 10.51 (range 6.87–21.56).
In the NSBBs group, propranolol was prescribed in 15 patients, and propranolol for 10 months was followed by carvedilol in 1 patient. The median dosage of propranolol was 20 mg (range 10–40 mg) per day, and the median duration of NSBBs was 1.56 years (range 0.58–6.15 years). In the no NSBBs group, 25 had never taken NSBB, 1 had taken NSBB for 1 month, and 1 had taken NSBB for 2 months. Additionally, in the no NSBBs group, 8 did not have previous gastrointestinal bleeding, of whom none had undergone endoscopic variceal treatment before; and 19 had previous gastrointestinal bleeding, of whom 12 had endoscopic variceal treatment and 7 did not have previous endoscopic variceal treatment.
The duration between baseline and follow-up contrast-enhanced CT/MRI scans (P = 0.214), grade of esophageal varices (P = 0.297), history of bleeding (P = 0.911), history of esophageal variceal treatment (P = 0.252), and esophageal variceal treatment at the first admission (P = 0.416) were all not significantly different between the NSBBs and no NSBBs groups.
Dynamic Changes of PVT
At the first admission, there were 16 patients diagnosed with PVT, including 8 in the NSBBs group and 8 in the no NSBBs group. The commonest location of PVT was MPV (56.2%) and the confluence of MPV and SV (50.0%), followed by SMV (31.2%), SV (25.0%), LPV (25.0%), and RPV (18.8%). The commonest degree of PVT was partial PVT (62.5%), followed by mural PVT (43.8%), complete PVT (12.5%), and fibrotic cord (6.2%).
Among the 16 patients with PVT at the first admission, 12 still had PVT at the last admission. Among the 27 without PVT at the first admission, 5 patients developed de novo PVT at the last admission (Fig. 3). Therefore, there were 17 patients diagnosed with PVT at the last admission, including 8 in the NSBBs group and 9 in the no NSBBs group. The commonest location of PVT was MPV (82.4%), followed by the confluence of MPV and SV (52.9%), RPV (47.1%), SMV (41.2%), SV (29.4%), and LPV (23.5%). The commonest degree of PVT was mural PVT (64.7%), followed by partial PVT (52.9%), complete PVT (41.2%), and fibrotic cord (23.5%).
Progressing Thrombosis
Thirteen patients had progressing thrombosis. The incidence of progressing thrombosis was significantly higher in the NSBBs group than in the no NSBBs group [50.0% (8/16) vs. 18.5% (5/27), P = 0.030]. Univariate logistic regression analyses also revealed that the use of NSBBs (OR 4.400, 95% CI 1.107–17.482, P = 0.035) was significantly associated with progressing thrombosis (Table 2). Multivariate logistic regression analyses revealed that the use of NSBBs was not independently associated with progressing thrombosis (OR 4.084, 95% CI 0.488–34.158, P = 0.194) (Table 2).
Discussion
Our study demonstrated a higher incidence of progressing thrombosis in cirrhotic patients who received NSBBs than in those who did not receive NSBBs, suggesting that NSBBs might facilitate the progression of PVT in cirrhosis. It has several distinct features and advantages. First, only contrast-enhanced CT/MRI scans, rather than ultrasound, were employed to evaluate the presence and severity of PVT. Our group discussed the dynamic change of PVT by reviewing every image and achieved a final decision about the outcome of PVT. Second, the information regarding the type, dosage, duration, and adherence of NSBBs were comprehensively reviewed by telephone communications with the patients and their relatives. Third, the use of NSBBs was strictly defined as a continuous use of NSBBs for at least 6 months and vice versa. This is primarily because one study potentially suggested a dose-dependent relationship of NSBBs with PVT [21]. Fourth, a composite endpoint of progressing thrombosis, including de novo PVT and aggravation of PVT, was employed. The limitation of this study is primarily attributable to the inclusion of only a relatively small number of patients according to the strict eligibility criteria.
Except for primary and secondary prophylaxis of variceal bleeding [12,13,14], recent evidence has further suggested the benefits of NSBBs in preventing the development of decompensated events in compensated patients [22], and the recurrence of esophageal varices after variceal eradication [23]. However, its detrimental effects in patients with acute kidney injury or spontaneous bacterial peritonitis have led to the proposal of a window for the use of NSBBs [24]. Evidence from our recent meta-analysis and the present observational study further suggest an increased risk and severity of PVT by NSBBs.
Several comparative studies have evaluated the outcomes of cirrhotic patients with PVT who were treated and not treated with anticoagulation therapy. In a study by Chen et al., in which 16 patients performed CT scans twice but did not receive anticoagulants, the thrombus was aggravated in 37.5% (6/16) of patients [25], which is quite similar with our findings that the incidence of aggravation of PVT was 37.5% in the no NSBBs group. However, in another study by Senzolo et al., the incidence of aggravation of PVT was 71.4% (15/21) [26]. Such a difference might be attributed to the fact that the severity of liver cirrhosis, such as higher MELD score [9] and Child–Pugh class B + C [27], are associated with the development of PVT. In the latter study, among the 21 patients, 16 had Child–Pugh class B + C [26].
Anticoagulation therapy can prevent the development [28], limit the aggravation [29, 30], and improve the recanalization of PVT [29, 30]. However, the beneficial effect of anticoagulants for preventing thrombus aggravation related to NSBBs is still unclear. In a cohort study by Pettinari et al., 81 patients took anticoagulants, of whom 60 also took NSBBs, and only 8 (9.9%) of them had aggravation of PVT [31]. Certainly, based on these preliminary observations, the efficacy of anticoagulants in patients treated with NSBBs cannot be concluded. Additionally, anticoagulation-related hemorrhage in cirrhosis is another major concern for physicians. Further studies are needed to fully weigh the efficacy of anticoagulants in cirrhotic patients treated with NSBBs.
Conclusions
Our study adds independent evidence that NSBBs are associated with the development and/or aggravation of PVT in patients with cirrhosis. Regardless, we have to acknowledge that the effect of NSBBs on progressing thrombosis seems to be mild, despite being significant in univariate analysis. Our study was not designed to assess causality, although a causal role would be concerning, and therefore large-scale well-designed cohort studies are warranted to confirm our findings and further explore the benefit and risk of prophylactic anticoagulation for PVT in patients treated with NSBBs.
References
Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749–61.
Sarin SK, Choudhury A, Sharma MK, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13:353–90.
Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy-definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716–21.
EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397–417.
Tarantino G, Citro V, Conca P, et al. What are the implications of the spontaneous spleno-renal shunts in liver cirrhosis? BMC Gastroenterol. 2009;9:89.
Qi X, Han G, Fan D. Management of portal vein thrombosis in liver cirrhosis. Nat Rev Gastroenterol Hepatol. 2014;11:435–46.
Cool J, Rosenblatt R. Portal vein thrombosis prevalence and associated mortality in cirrhosis in a nationally representative inpatient cohort. J Gastroenterol Hepatol. 2019;34:1088–92.
Nery F, Chevret S, Condat B, et al. Causes and consequences of portal vein thrombosis in 1,243 patients with cirrhosis: results of a longitudinal study. Hepatology. 2015;61:660–7.
Abdel-Razik A, Mousa N, Elhelaly R, Tawfik A. De-novo portal vein thrombosis in liver cirrhosis: risk factors and correlation with the Model for End-stage Liver Disease scoring system. Eur J Gastroenterol Hepatol. 2015;27:585–92.
Stine JG, Wang J, Shah PM, et al. Decreased portal vein velocity is predictive of the development of portal vein thrombosis: a matched case-control study. Liver Int. 2018;38:94–101.
Zocco MA, Di Stasio E, De Cristofaro R, et al. Thrombotic risk factors in patients with liver cirrhosis: correlation with MELD scoring system and portal vein thrombosis development. J Hepatol. 2009;51:682–9.
EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–60.
Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310–35.
de Franchis R. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–52.
Cioni G, D’Alimonte P, Zerbinati F, et al. Duplex–Doppler ultrasonography in the evaluation of cirrhotic patients with portal hypertension and in the analysis of their response to drugs. J Gastroenterol Hepatol. 1992;7:388–92.
Baik SK, Park DH, Kim MY, et al. Captopril reduces portal pressure effectively in portal hypertensive patients with low portal venous velocity. J Gastroenterol. 2003;38:1150–4.
Zoli M, Marchesini G, Brunori A, Cordiani MR, Pisi E. Portal venous flow in response to acute beta-blocker and vasodilatatory treatment in patients with liver cirrhosis. Hepatology. 1986;6:1248–51.
Qi X, Bai M, Fan D. Nonselective beta-blockers may induce development of portal vein thrombosis in cirrhosis. World J Gastroenterol. 2014;20:11463–6.
Xu X, Guo X, De Stefano V, et al. Nonselective beta-blockers and development of portal vein thrombosis in liver cirrhosis: a systematic review and meta-analysis. Hepatol Int. 2019;13:468–81.
Peng Y, Qi X, Guo X. Child–Pugh versus MELD score for the assessment of prognosis in liver cirrhosis: a systematic review and meta-analysis of observational studies. Medicine (Baltimore). 2016;95:e2877.
Nery F, Correia S, Macedo C, et al. Nonselective beta-blockers and the risk of portal vein thrombosis in patients with cirrhosis: results of a prospective longitudinal study. Aliment Pharmacol Ther. 2019;49:582–8.
Villanueva C, Albillos A, Genesca J, et al. beta blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2019;393:1597–608.
Xu X, Guo X, Tacke F, Shao X, Qi X. Use of nonselective beta blockers after variceal eradication in cirrhotic patients undergoing secondary prophylaxis of esophageal variceal bleeding: a critical review of current evidence. Ther Adv Chronic Dis. 2019;10:2040622319862693.
Mandorfer M, Bota S, Schwabl P, et al. Nonselective beta blockers increase risk for hepatorenal syndrome and death in patients with cirrhosis and spontaneous bacterial peritonitis. Gastroenterology. 2014;146(1680–90):e1.
Chen H, Liu L, Qi X, et al. Efficacy and safety of anticoagulation in more advanced portal vein thrombosis in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2016;28:82–9.
Senzolo M, Sartori T, Rossetto V, et al. Prospective evaluation of anticoagulation and transjugular intrahepatic portosystemic shunt for the management of portal vein thrombosis in cirrhosis. Liver Int. 2012;32:919–27.
Violi F, Corazza GR, Caldwell SH, et al. Portal vein thrombosis relevance on liver cirrhosis: Italian venous thrombotic events registry. Intern Emerg Med. 2016;11:1059–66.
Villa E, Camma C, Marietta M, et al. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology. 2012;143(1253–60):e4.
Loffredo L, Pastori D, Farcomeni A, Violi F. Effects of anticoagulants in patients with cirrhosis and portal vein thrombosis: a systematic review and meta-analysis. Gastroenterology. 2017;153(480–7):e1.
Qi X, De Stefano V, Li H, Dai J, Guo X, Fan D. Anticoagulation for the treatment of portal vein thrombosis in liver cirrhosis: a systematic review and meta-analysis of observational studies. Eur J Intern Med. 2015;26:23–9.
Pettinari I, Vukotic R, Stefanescu H, et al. Clinical impact and safety of anticoagulants for portal vein thrombosis in cirrhosis. Am J Gastroenterol. 2019;114:258–66.
Acknowledgements
We thank the participants of the study, including Han Deng, Ran Wang, Jing Li, Yingying Li, Xiangbo Xu, Zhaohui Bai, Qianqian Li, Kexin Zheng, Le Wang, and Fangfang Yi in our study team, for their efforts in setting up and updating the prospective database.
Funding
The study was partially supported by the Science and Technology Project Foundation of Shenyang (19-112-4-005). No Rapid Service Fee was received by the journal for the publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Conceptualization: XQ; Methodology: XX and XQ; Formal analysis: XX and XQ; Investigation: XQ; Resource: XX and XQ; Data curation: XX, SX, YH, FY, and XQ; Writing-original draft: XX and XQ; Writing-review and editing: MP, VDS, DV, and XQ; Visualization: XX and XQ; Supervision: XG and XQ; Project administration: XQ. All authors have made an intellectual contribution to the manuscript and approved the submission.
Disclosures
Xiangbo Xu, Shixue Xu, Massimo Primignani, Valerio De Stefano, Yanglan He, Fangfang Yi, Xiaozhong Guo, Dominique Valla, and Xingshun Qi have nothing to disclose.
Compliance with Ethics Guidelines
The study was conducted in accordance with the Helsinki Declaration and approved by the Medical Ethical Committee of the General Hospital of Northern Theater Command. Due to the retrospective observational nature of the study, the requirement for patient consent was waived.
Data Availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.11770686.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Xu, X., Xu, S., Primignani, M. et al. Nonselective β-Blockers May Progress the Thrombosis of Portal Venous System in Cirrhotic Patients: A Retrospective Observational Study. Adv Ther 37, 1452–1463 (2020). https://doi.org/10.1007/s12325-020-01250-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-020-01250-z