Abstract
Introduction
Intravenous (IV) iron is typically the preferred treatment for patients with iron deficiency anemia (IDA) who cannot tolerate or absorb oral iron, or who require fast replenishment of iron stores pre-operatively. Several IV iron formulations are available with different dosing characteristics affecting infusion speed and maximum dose. The aim was to develop a resource impact model to calculate the cost of establishing an IV iron clinic and model resource impact of different IV irons to inform clinicians and service providers implementing innovative pre-operative IV iron services in Ireland.
Methods
A resource impact tool was developed to model resource utilization and IDA treatment costs. Two fast-administration, high-dose formulations of IV iron are available in Ireland: iron isomaltoside 1000/ferric derisomaltose (IIM) and ferric carboxymaltose (FCM). The tool modeled clinic throughput based on their different dosing characteristics in a specific IDA population, capturing fixed overheads, variable costs, clinic income from private and publicly-funded patients, and savings associated with IV iron.
Results
Based on a 70:30 split between public and private patients in a new pre-operative service with capacity for 12 infusion slots weekly, IIM would facilitate correction of iron deficits in 474 patients annually, resulting in a net annual clinic balance of €42,736 on income of €159,887 and net costs of €117,151. FCM would facilitate treatment of 353 patients, resulting in a net annual clinic balance of €36,327 on income of €116,050 and costs of €79,722, a difference of €6408 and 121 patients treated in favor of using IIM over FCM.
Conclusion
Based on this provider-perspective analysis, IIM would maximize clinic throughput relative to other IV iron formulations, allowing clinicians in Ireland to optimize their current service provision and expenditure, and model the impact of introducing IV iron clinics for pre-operative patients with IDA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The benefits of using intravenous (IV) iron to correct pre-operative iron deficiency anemia (IDA) have been demonstrated in numerous randomized controlled trials and include shortening the length of hospital stay, reducing red blood cell transfusion rates and improving quality of life. |
Two fast-administration, high-dose IV iron formulations are currently available in Ireland: ferric carboxymaltose (Ferinject® or FCM) and iron isomaltoside 1000/ferric derisomaltose (Monover® or IIM). |
A resource impact model was used to evaluate resource utilization and pre-operative IDA treatment costs in Ireland based on a combination of real-world data from an infusion clinic at Cork University Hospital, previously published models of IDA treatment, and the posological characteristics of modern IV iron formulations, including FCM and IIM. |
Based on clinic throughput modeling using data from an existing IV iron clinic in Ireland, and the posological characteristics of two IV iron formulations as specified in the summaries of product characteristics, IIM was shown to result in substantially higher patient throughput than other IV iron formulations. |
.
Introduction
Anemia is defined by the World Health Organization as hemoglobin levels < 13 g/dL in men older than 15 years, < 12 g/dL in non-pregnant women older than 15 years, and < 11 g/dL in pregnant women [1]. Iron deficiency is the top-ranking cause of anemia worldwide, and iron deficiency anemia (IDA) is the combination of anemia and depressed total body iron, with the latter typically confirmed by low (< 30 μg/L) serum ferritin levels [2]. In Ireland in 2013, Kassebaum et al. estimated that there were 464,893 patients with mild anemia, 352,789 patients with moderate anemia, and 23,470 patients with severe anemia, with IDA comprising 62.6% of all cases of anemia globally [3].
Anemia has multiple etiologies, including a wide range of conditions that cause diminished iron uptake, increased iron demand or increased iron loss, with gastrointestinal or gynecological involvement being among the most common causes [4]. For a variety of pathophysiological reasons including inflammatory processes, nutritional deficiencies, and iatrogenic causes, anemia is also extremely common in patients admitted to hospital, including those undergoing elective surgery [5]. For instance, in a cohort of 232,450 adult patients hospitalized in the Cleveland Clinic Health System between January 2009 and August 2011, 43,741 (19%) presented with anemia upon admission, and 60% of those who were not anemic upon presentation developed hospital-acquired anemia [6].
In patients undergoing surgery, the prevalence of anemia varies by the nature of the disease in the patient population; for instance, around one-third of patients undergoing non-cardiac surgery have pre-operative anemia [7, 8], while studies in colorectal surgery have shown even higher rates, with 57.6% of patients with right-sided colon cancer having anemia prior to surgical resection [9]. Anemia is considered to be a symptom of the underlying condition requiring surgery, but several large studies have recently established that pre-operative anemia is also an independent risk factor for increased perioperative morbidity, mortality, and prolonged length of hospital stay [7, 10,11,12]. Pre-operative anemia increases the likelihood of perioperative allogeneic blood transfusion and the associated risks of wound problems, systemic sepsis, and prolonged length of post-operative hospital stay [5, 13,14,15].
Correction of pre-operative anemia can therefore play an important role in reducing the risk of these perioperative complications (including mortality), shortening the length of hospital stay, reducing red blood cell (RBC) transfusion rates and improving quality of life. This has been demonstrated in smaller randomized clinical trials, in real-world data from large cohorts of patients, and in meta-analyses [16, 17]. Randomized controlled trials (RCTs) have further illustrated the benefits of using intravenous (IV) iron prior to surgery. In 2016, an RCT in 72 patients published by Froessler and colleagues showed a 60% reduction in the transfusion rate of allogeneic RBCs (12.5% vs. 31.25%), a greater increase in hemoglobin levels (+ 0.8 g/dL vs. + 0.1 g/dL), and a shorter length of hospital stay (7.0 days vs. 9.7 days) in patients treated with IV iron relative to those receiving usual care [18]. Spahn et al. also recently reported the findings of an RCT in patients with IDA prior to cardiac surgery, in which 20 mg/kg of IV iron, 40,000 U of subcutaneous erythropoietin alpha, 1 mg subcutaneous vitamin B12, and 5 mg oral folic acid were compared with placebo [19]. The study showed a significant reduction in the median number of red blood cell units transfused from 1 to 0, with a corresponding increase in hemoglobin concentration, reticulocyte count, and a reticulocyte hemoglobin content [19]. Similarly, a 2018 study by Biboulet and colleagues demonstrated the superior efficacy of IV iron in combination with erythropoietin relative to oral iron in patients undergoing major orthopedic surgery, showing a 0.7-g/dl larger increase in preoperative hemoglobin (p < 0.001) [20].
Reductions in RBC transfusion rates associated with correction of pre-operative anemia mean that IV iron infusion plays a central role in patient blood management (PBM) programs. In 2015, this role was codified in UK guidance from the National Institute for Health and Care Excellence (NICE). The guidance focused on reducing unnecessary blood transfusions, with IV iron being recommended as an alternative to blood, especially in patients with IDA requiring rapid iron replenishment, intolerant of oral iron, or with functional iron deficiency [21]. In Ireland, PBM initiatives, such as improving blood stock management and increasing monitoring of RBC usage indicators, have resulted in remarkable reductions in RBC issuance [22]. Increasing the use of IV iron in the correction of pre-operative anemia therefore represents an additional initiative that has the potential to still further improve the outcomes of the PBM program in Ireland.
More recently, a randomized trial in patients with colorectal cancer was published by Keeler et al., demonstrating that ferric carboxymaltose (FCM; Ferinject®; Vifor France, Paris, France) administered 2 weeks prior to surgery significantly increased hemoglobin levels on the day of surgery and 3 months after surgery, and significantly improved quality of life relative to oral iron. No difference in complications, transfusion or length of stay was demonstrated, which may be due to sample size, and/or the patients having non-metastatic cancer, with a relatively good condition and a low risk profile (ASA score). However, the group receiving intravenous iron needed a dose of 1500–2000 mg of intravenous iron in 82 of the cases, meaning that two hospital visits were required to receive the full dose, since only 1000 mg of FCM can be given in a single infusion [23, 24].
Further to this research, the ITACS and PREVENTT double-blind RCTs are currently ongoing [25,26,27]. The primary endpoint of ITACS is the number of days alive and out of hospital in the first 30 days after surgery in patients receiving placebo versus those receiving 1000 mg of IV iron [25, 26]. Secondary endpoints include the proportion of patients experiencing correction of anemia, number of allogeneic blood units transfused, intensive care and hospital stay duration, quality of life, and cost-effectiveness [25]. Similarly, PREVENTT is investigating the risk of blood transfusion or death, and the blood transfusion rate in patients with anemia prior to undergoing elective major (> 1 h) open abdominal surgery [27]. Patients (n = 487) were randomly assigned to receive either placebo or IV iron prior to surgery. Once completed, PREVENTT will represent the largest RCT ever conducted on the efficacy of correcting pre-operative anemia.
Two fast-administration, high-dose IV iron formulations are currently available in Ireland: FCM and iron isomaltoside 1000/ferric derisomaltose (IIM; Monover®; Pharmacosmos, Holbæk, Denmark). Both iron formulations are colloidal, consisting of iron (III) hydroxide complexed with different carbohydrates. The drugs differ in their approved posology: IIM can be dosed up to 20 mg per kg body weight with no other dose limitations, while FCM can only be dosed up to a maximum of 1000 mg and 20 mg per kg body weight per infusion.
While the benefits of pre-operative IV iron infusion have been demonstrated in the Froessler et al. RCT [18], the economic and logistical aspects of pre-operative iron infusion have not been evaluated. In the present study, we therefore sought to develop a model to evaluate the resource use and cost implications associated with establishing an iron infusion clinic for the treatment of pre-operative IDA in Ireland, with the secondary objective of quantifying how the use of different IV iron formulations affects resource use and costs in the clinic setting.
Methods
Resource Impact Model
A resource impact model was developed in Microsoft Excel (Microsoft, Redmond, WA, USA) to model resource utilization and pre-operative IDA treatment costs based on a combination of infusion costing data from the finance department at Cork University Hospital, previously-published models of IDA treatment, and the posological characteristics of modern IV iron formulations [28]. A multidisciplinary team was involved in establishing key aspects of the financial flows and resource utilization in an iron infusion clinic in Ireland, including fixed overhead costs, variable costs (i.e., costs incurred with each infusion), clinic income from private and publicly-funded patients, and savings associated with the correction of pre-operative anemia (Fig. 1; Table 1).
The resource impact model simulated clinic throughput based on an assumed number of available infusion slots per year, with a model input to govern the allocation of infusion slots to privately- and publicly-funded patients, and elective versus non-elective procedures. The clinic throughput assumptions were combined with a model of IV iron dosing characteristics and iron deficits in a specific population with pre-operative IDA to establish the number of patients whose iron deficiency could be corrected in the clinic per year.
Iron deficits in the modeled cohort were based on the simplified tables of iron need found in the summaries of product characteristics (SPCs) for the respective IV iron products [29, 30]. The simplified tables of iron need were used in preference to the alternative iron deficit calculation approach of the Ganzoni equation, on the grounds that the European Crohn’s and Colitis Organisation guidelines recommend the use of this calculation method [31].
The use of the Ganzoni formula as the basis of the iron deficit model was explored in a sensitivity analysis:
In addition to modeling IIM and FCM, resource use and costs associated with two other IV iron products were also modeled: iron sucrose (IS; Venofer®; Vifor France), which can be dosed up to 200 mg per infusion, and low molecular weight iron dextran (LMWID; CosmoFer®; Pharmacosmos), which can be infused intravenously over 4–6 h at up to 20 mg/kg.
Base Case Analysis
In the base case analysis, it was assumed that the clinic would operate for half a day (4 h) a week with 12 infusion slots per half-day, corresponding to 624 infusion slots per annum. The assumption of 12 infusion slots was based on an assumption of one nurse being capable of administering and monitoring three infusions per hour. For each half-day of clinic operation, a nurse was assumed to be present 100% of the time along with a member of medical admin staff, with a consultant also present 60% of the time (2.4 h per half day). The analysis was conducted over a 1-year time horizon from the perspective of an infusion clinic in Ireland.
Clinic running costs were taken from a variety of sources specific to the Irish setting (Table 2). Staff costs and costs associated with diagnostic tests, cannulas, dressings, and giving sets were provided by Cork University Hospital, while costs of IV iron were based on list prices from MIMS Ireland in January 2019.
Cost savings arising from the correction of pre-operative anemia with IV iron were based on unit cost estimates from Cork University Hospital (Table 3). In the absence of resource use data specific to Ireland, Irish unit costs associated with reduced ward or intensive care unit (ICU) stays were combined with Hospital Episode Statistics (HES) from NHS Digital based on data from all NHS hospitals in England [32]. Savings arising from a reduction in the need for RBC transfusions were based on the difference in the number of units transfused intra- and post-operatively in patients receiving pre-operative iron (8 units in 40 patients) versus those receiving usual care (25 units in 32 patients) in the Froessler et al. RCT [18].
Estimates of clinic income for publicly-funded day case admissions, private day case admissions, private IV iron pass-through costs, and private billing for materials and diagnostic test costs were provided by Cork University Hospital. For the base case analysis, a public:private split of 70:30 was assumed based on consultation with Cork University Hospital, and an elective:non-elective split of 77.4:22.6 was assumed based on the NHS HES data [32].
The characteristics of the specific IDA population were based on a weighted average of mean baseline hemoglobin and bodyweight from seven studies included in a recent review of IDA [33]. Mean baseline hemoglobin was taken to be 9.99 g/dL with a standard deviation of 1.03 g/dL, and bodyweight was assumed to be 82.36 kg with a standard deviation of 22.5 kg. The modeled cohort was distributed across lognormal distributions of hemoglobin and bodyweight to reflect the right-skewed distributions typically observed of both bodyweight and hemoglobin at a population level (Fig. 2) [34, 35]. To avoid capturing patients for whom no specific dose recommendations are made in the dosing tables in the SPCs, the lower end of the bodyweight distribution was truncated at 50 kg by mirroring the probability density function around the minimum weight of 50 kg, as described previously [28]. Note that the analysis was based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Sensitivity Analyses
A series of one-way sensitivity analyses were conducted around key model inputs to establish the effect of individual parameters on top-line outputs. Analyses were conducted in which 100% of procedures were modeled as being elective and 100% being non-elective; the assumed clinic throughput was varied from 3 infusions per hour in the base case analysis to 2 and 4 infusions per hour; the bodyweight and hemoglobin levels of the population with IDA were adjusted to match the baseline characteristics in the Froessler et al. RCT [18]; the Ganzoni equation was used to model iron need; the assumptions of reduced ward stay and hospital readmission in the base case were abolished; and the proportion of total infusion time overseen by a consultant was increased from 60% in the base case to 100%.
Results
Based on the assumptions employed in the base case analysis, the resource impact model reported the number of privately- and publicly-funded patients whose pre-operative IDA would be corrected given a fixed annual clinic throughput in terms of the number of iron infusions. The model also reported the fixed and variable clinic running costs, the cost savings arising from correcting pre-operative anemia, and the clinic income from conducting a mix of privately- and publicly-funded day cases.
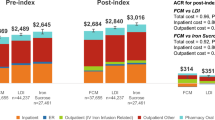
Based on the simplified tables of iron need in a new pre-operative service with capacity for 624 infusions annually, IIM would facilitate correction of iron deficits in 474 patients, compared with 353 for FCM, 81 for IS, and 158 for LMWID (Fig. 3a). Based on a 70:30 split between public and private patients and a 77.4:22.6 split between elective and non-elective iron replacement, the majority (54%) of patients underwent publicly-funded elective iron replacement, with 23% undergoing elective privately-funded replacement, and 16% and 7% undergoing non-elective iron replacement with public and private funding, respectively (Fig. 3b).
a Patients with pre-operative iron deficiency anemia treatable annually with 12 infusion slots per week (624 per year) based on simplified dosing tables and assuming lognormal distributions of hemoglobin and bodyweight, and b proportions of publicly- versus privately-funded patients and proportions treated as elective versus non-elective
The use of IIM resulted in the treatment of 474 patients and a net annual clinic balance of €42,736 on income of €159,887, costs of €258,850 and savings of €141,699 relative to not addressing pre-operative anemia (Fig. 4). FCM would facilitate treatment of 353 patients, resulting in a net annual clinic balance of €36,327 on income of €116,050, costs of €185,036 and savings of €105,314 relative to not correcting pre-operative anemia. Relative to FCM, IIM would therefore result in an additional surplus balance of €6408 over 1 year and 121 (34.2%) additional patients treated with IV iron. The low throughput with IS resulted in lower income, variable costs, savings and net clinic balance; income was projected to be €23,242, with costs of €37,125, and annual savings relative to not correcting pre-operative anemia of €24,077, resulting in a net clinic balance of €10,194. The slower rate of infusion of LMWID similarly resulted in lower throughput with income of €43,371, costs of €58,201, and savings of €47,233 resulting in a net clinic balance of €32,403.
One-way sensitivity analyses showed that the net clinic balance was most sensitive to the number of infusion slots available per half-day; higher throughputs resulted in an increased net balance as fixed clinic running costs per procedure decreased while clinic income increased (Table 4). Changing the iron need calculation approach to the Ganzoni equation resulted in the largest change in the difference in net clinic balance and patient throughput between the iron formulations, with the difference between IIM and FCM increasing from €6408 (and an additional 121 patients) in the base case to €15,963 (and an additional 191 patients) using the Ganzoni equation. Changing the baseline cohort characteristics to match those from the Froessler et al. study [18] also had a notable effect on the difference between formulations, increasing the difference in net clinic balance between IIM and FCM to €12,217 and increasing the difference in patient throughput to 169 patients (520 vs. 352) from 121 in the base case.
Discussion
The present analysis showed that, in an iron infusion clinic with a fixed annual throughput, IIM would result in more patients with IDA receiving treatment to correct their iron deficiency. In addition to the increased throughput, the use of IIM was projected to result in a higher net clinic balance relative to FCM, IS and LMWID. The analysis represents the first attempt to characterize the costs of running an IV iron clinic in the Irish setting. One previous study, by Radia and colleagues, was identified in the literature that evaluated the selection of IV iron in an IV iron service in the United Kingdom, but the selection of IV iron was based on contraindications for IIM which have since been removed from the IIM SPC, and did not factor in the special warnings and precautions now contained in the FCM SPC pertaining to hypophosphatemia and hepatic or renal impairment [36]. Other studies have previously modeled costs of performing infusions in other healthcare settings and indications, focusing on the administration of biological agents, such as infliximab in patients with inflammatory bowel disease and rheumatoid arthritis, alongside other biologic disease-modifying antirheumatic drugs such as rituximab and abatacept [37, 38].
The comparative analysis of the modern IV iron formulations was driven primarily by posological differences between the iron formulations. Using the simplified tables of iron need, all patients weighing ≥ 70 kg and all patients with hemoglobin levels < 10 g/dL cannot be infused with a sufficiently high dose (i.e. 1,500 mg or 2000 mg) of FCM to provide the recommended amount of iron to correct the iron deficit in a single infusion. The weight-dependent maximum dosing of IIM means a single infusion of IIM is sufficient to administer the recommended dose (1500 mg) in all patients with hemoglobin ≥ 10 g/dL weighing 75 kg or more. The limitations of dosing with FCM in the PBM context were borne out in the Keeler et al. trial in patients with pre-operative anaemia, in which 82% of patients required two iron infusions prior to surgery for colorectal cancer [23]. Conversely, recent real-world data from the UK suggests that, where a majority of patients require > 1000 mg of iron, IIM enables administration in a single dose [39].
The relative efficacy of the two IV iron formulations should also be considered when interpreting the findings of the analysis. A recent network meta-analysis conducted by Aksan et al. reported that “concerning efficacy, no statistically significant difference was found when comparing FCM, [IIM] and IS”, specifically with regard to the proportion of patients showing hematopoietic response, defined as normalization of hemoglobin levels or an increase in Hb of ≥ 2 g/dL in patients with IBD [40]. While the authors subsequently noted that “[t]hese types of analyses are more exploratory-pragmatic or ‘observational’ rather than confirmatory” [41], the finding of no significant difference in the proportion of responders is aligned with a recent indirect treatment comparison (ITC) of IIM and FCM using a common comparator of IS. The ITC reported no significant difference in the proportion of patients achieving a clinically-relevant response with IIM and FCM, but did report a significantly larger mean increase in hemoglobin from baseline with IIM relative to FCM [42].
Notably, the average modeled dose of 1493 mg in the present study is high relative to real-world studies such as the non-interventional Monofer (NIMO) study, which reported a mean administered iron dose of 1100 mg in 185 patients with anemia [43]. As reported in the study, however, the mean iron need was calculated to be 1481 mg according to the simplified table of iron need and 1324 mg according to the Ganzoni formula. The NIMO study authors acknowledged the shortfall of iron administered in clinical practice in their discussion, but did not investigate or speculate on the underlying reason [43]. Regardless of the reason, the shortfall was not without consequence: of the patients enrolled in the NIMO study with anemia at baseline, 37% were still anemic following the first iron treatment, suggesting that the administered dose was indeed insufficient [41]. Notably, patients in NIMO receiving doses of IIM over 1000 mg experienced a 65% lower probability of needing retreatment versus patients receiving exactly 1000 mg, illustrating a dose–response relationship that would justify administering higher initial doses to reduce the need for retreatment [41].
High doses of IIM have been shown to be both well tolerated and effective in the PROMISE trial, in which 21 patients with IBD patients and IDA were treated with single doses of up to 2000 mg, and cumulative doses of between 1500 and 3000 mg of IV iron [44]. In PROMISE, no serious adverse drug reactions were observed, and no patients experienced severe hypophosphatemia (s-phosphate level < 1 mg/dL) [44]. While the sample size in PROMISE was small, the absence of serious adverse drug reactions suggests that there should be no additional concern associated with administering > 1000 mg versus administering 1000 mg or less. Recent evidence has also shown that the risk of hypophosphatemia appears to be significantly greater with FCM relative to IIM, with a pooled analysis of 245 patients in the two PHOSPHARE RCTs showing severe hypophosphatemia occurring in 11.3% of patients treated with FCM versus 0.0% of patients treated with IIM (p < 0.0001), and hypophosphatemia < 2 mg/dL occurring in 74.4% of patients treated with FCM versus 8.0% treated with IIM (p < 0.0001) [45].
Finally, it is worth considering the limitations of the present study when interpreting the findings. The first notable limitation is that the clinic running costs were not exhaustive; for instance, some overhead costs such as depreciation, ground rent or space charges, maintenance costs, and utility bills were not included. A second important limitation was the exclusion of any clinical discretion on the administered iron doses. For instance, the simplified tables of iron need for both IIM and FCM recommend that a patient weighing 72 kg with a hemoglobin level of 10 g/dL would require 1500 mg. In the case of IIM, such a patient would receive one dose of 1440 mg in a first infusion (the maximum permissible dose based on 20 mg/kg) and then 60 mg in a second infusion. In practice, the clinician may decide that the first infusion would be sufficient to address the iron deficit and that no subsequent infusion would be required. However, this modeling assumption results in a conservative estimate of the net clinic balance by reducing patient throughput for a given number of infusion slots, and was adopted for all iron formulations. Given the more fine-grained bodyweight-based dosing increments with IIM relative to FCM (and therefore the increased frequency with which this assumption would affect IIM), the assumption may have also underestimated the benefit of IIM versus FCM. One final potential limitation is the generalizability of the model and findings. The study deliberately adopted the perspective of a pre-operative infusion clinic in Ireland to address our specific research question. The clinic perspective, focusing on the net clinic balance, is notably distinct from national payer perspectives where diagnosis-related groups, healthcare resource groups, or reference costs are commonly used to establish the size of payments to service providers. Nevertheless, the modeling approach employed should be applicable to infusion clinics in other geographies and payer settings with only minimal changes.
Conclusions
Treating IDA before surgery is a cornerstone of PBM as recommended by NICE, and has been shown to improve patient outcomes and save costs at a large scale. The development of a robust resource impact model in the present analysis allows decision-makers in Ireland to model the impact of introducing IV iron clinics for the treatment of pre-operative patients with IDA.
Based on clinic throughput modeling using data from an existing IV iron clinic in Ireland, and the posological characteristics of the IV iron formulations as specified in the SPCs, IIM was shown to result in substantially higher patient throughput than other IV iron formulations, while also driving favorable economic outcomes from the clinic perspective. IIM should therefore be considered as the IV iron formulation of choice in the establishment of IV iron infusion clinics for the treatment of pre-operative anemia in Ireland.
References
Goddard AF, James MW, McIntyre AS, et al. Guidelines for the management of iron deficiency anaemia. Gut. 2011;60:1309–16.
Camaschella C. Iron-deficiency anemia. New Engl J Med. 2015;372:1832–43.
Kassebaum NJ. The global burden of anemia. Hematol Oncol Clin North Am. 2016;30(2):247–308.
Jimenez K, Kulnigg-Dabsch S, Gasche C. Management of iron deficiency anemia. Gastroenterol Hepatol (N Y). 2015;11(4):241–50.
Muñoz M, Gómez-Ramírez S, Campos A, Ruiz J, Liumbruno GM. Pre-operative anaemia: prevalence, consequences and approaches to management. Blood Transfus. 2015;13(3):370–9.
Koch CG, Li L, Sun Z, Hixson ED, Tang AS, Phillips SC, Blackstone EH, Henderson JM. From bad to worse: anemia on admission and hospital-acquired anemia. J Patient Saf. 2017;13(4):211–6.
Musallam KM, Tamim HM, Richards T, Spahn DR, Rosendaal FR, Habbal A, Khreiss M, Dahdaleh FS, Khavandi K, Sfeir PM, Soweid A, Hoballah JJ, Taher AT, Jamali FR. Preoperative anaemia and postoperative outcomes in non-cardiac surgery: a retrospective cohort study. Lancet. 2011;378(9800):1396–407.
Dunne JR, Malone D, Tracy JK, Gannon C, Napolitano LM. Perioperative anemia: an independent risk factor for infection, mortality, and resource utilization in surgery. J Surg Res. 2002;102(2):237–44.
Dunne JR, Gannon CJ, Osborn TM, Taylor MD, Malone DL, Napolitano LM. Preoperative anemia in colon cancer: assessment of risk factors. Am Surg. 2002;68(6):582–7.
Wu WC, Schifftner TL, Henderson WG, Eaton CB, Poses RM, Uttley G, Sharma SC, Vezeridis M, Khuri SF, Friedmann PD. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. JAMA. 2007;297:2481–8.
Scrascia G, Guida P, Caparrotti SM, Capone G, Contini M, Cassese M, Fanelli V, Martinelli G, Mazzei V, Zaccaria S, Paparella D. Incremental value of anemia in cardiac surgical risk prediction with the European System for Cardiac Operative Risk Evaluation (EuroSCORE) II model. Ann Thorac Surg. 2014;98:869–75.
Ranucci M, Di Dedda U, Castelvecchio S, et al. Surgical and Clinical Outcome Research (SCORE) group impact of preoperative anemia on outcome in adult cardiac surgery: a propensity-matched analysis. Ann Thorac Surg. 2012;94:1134–41.
Elhenawy AM, Meyer SR, Bagshaw SM, MacArthur RG, Carroll LJ. Role of preoperative intravenous iron therapy to correct anemia before major surgery: study protocol for systematic review and meta-analysis. Syst Rev. 2015;4:29.
Leal-Noval SR, Muñoz-Gómez M, Jiménez-Sánchez M, Cayuela A, Leal-Romero M, Puppo-Moreno A, Enamorado J, Arellano-Orden V. Red blood cell transfusion in non-bleeding critically ill patients with moderate anemia: is there a benefit? Intensive Care Med. 2013;39:45–53.
Ferraris VA, Davenport DL, Saha SP, Austin PC, Zwischenberger JB. Surgical outcomes and transfusion of minimal amounts of blood in the operating room. Arch Surg. 2012;147(1):49–55.
Althoff FC, Neb H, Herrmann E, Trentino KM, Vernich L, Füllenbach C, Freedman J, Waters JH, Farmer S, Leahy MF, Zacharowski K, Meybohm P, Choorapoikayil S. Multimodal patient blood management program based on a three-pillar strategy: a systematic review and meta-analysis. Ann Surg. 2019;269(5):794–804.
Leahy MF, Hofmann A, Towler S, Trentino KM, Burrows SA, Swain SG, Hamdorf J, Gallagher T, Koay A, Geelhoed GC, Farmer SL. Improved outcomes and reduced costs associated with a health-system-wide patient blood management program: a retrospective observational study in four major adult tertiary-care hospitals. Transfusion. 2017;57(6):1347–58.
Froessler B, Palm P, Weber I, Hodyl NA, Singh R, Murphy EM. The important role for intravenous iron in perioperative patient blood management in major abdominal surgery: a randomized controlled trial. Ann Surg. 2016;264(1):41–6.
Spahn DR, Schoenrath F, Spahn GH, Seifert B, Stein P, Theusinger OM, Kaserer A, Hegemann I, Hofmann A, Maisano F, Falk V. Effect of ultra-short-term treatment of patients with iron deficiency or anaemia undergoing cardiac surgery: a prospective randomised trial. Lancet. 2019;393(10187):2201–12.
Biboulet P, Bringuier S, Smilevitch P, Loupec T, Thuile C, Pencole M, Maissiat G, Dangelser G, Capdevila X. Preoperative epoetin-α with Intravenous or oral iron for major orthopedic surgery: a randomized controlled trial. Anesthesiology. 2018;129(4):710–20.
National Institute for Health and Care Excellence. NICE Guideline 24: Blood transfusion. London, UK; 2019. https://www.nice.org.uk/guidance/ng24. Accessed Oct 22 2019.
Gombotz H, Hofmann A, Nørgaard A, Kastner P. Supporting patient blood management (PBM) in the EU. A Practical implementation guide for hospitals. European Commission; Brussels, Belgium; 2017. https://op.europa.eu/en/publication-detail/-/publication/93e1bbbf-1a8b-11e7-808e-01aa75ed71a1. Accessed Nov 8 2019.
Keeler BD, Simpson JA, Ng O, Padmanabhan H, Brookes MJ, Acheson AG, IVICA Trial Group. Randomized clinical trial of preoperative oral versus intravenous iron in anaemic patients with colorectal cancer. Br J Surg. 2017;104(3):214–21.
Keeler BD, Dickson EA, Simpson JA, Ng O, Padmanabhan H, Brookes MJ, Acheson AG, IVICA Trial Group. The impact of pre-operative intravenous iron on quality of life after colorectal cancer surgery: outcomes from the intravenous iron in colorectal cancer-associated anaemia (IVICA) trial. Anaesthesia. 2019;74(6):714–25.
Bayside health. Intravenous iron for treatment of anaemia before cardiac surgery (ITACS). Clinicaltrials.gov. NCT02632760. https://clinicaltrials.gov/ct2/show/NCT02632760. Accessed Nov 6 2019.
National Health Service. Health Research Authority. IV iron for treatment of anaemia before cardiac surgery. https://www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/itacs-trial/. Accessed Nov 8 2019.
Richards T, Clevenger B, Keidan J, Collier T, Klein AA, Anker SD, Kelly JD. PREVENTT: preoperative intravenous iron to treat anaemia in major surgery: study protocol for a randomised controlled trial. Trials. 2015;16:254.
Pollock RF, Muduma G. A budget impact analysis of parenteral iron treatments for iron deficiency anemia in the UK: reduced resource utilization with iron isomaltoside 1000. Clinicoecon Outcomes Res. 2017;9:475–83.
Health Products Regulatory Authority. Monover 100 mg/ml solution for injection/infusion (vials). Summary of product characteristics. Dublin, Ireland; 2019. https://www.hpra.ie/img/uploaded/swedocuments/LicenseSPC_PA0982-002-002_09022018100041.pdf. Accessed Oct 22 2019.
Health Products Regulatory Authority. Ferinject 50 mg iron/mL solution for injection/infusion. Summary of product characteristics. Dublin, Ireland; 2019. http://www.hpra.ie/img/uploaded/swedocuments/FINAL%20Licence_PA0949-004-001_01032019115628.pdf. Accessed Oct 22 2019.
Dignass AU, Gasche C, Bettenworth D, Birgegård G, Danese S, Gisbert JP, Gomollon F, Iqbal T, Katsanos K, Koutroubakis I, Magro F, Savoye G, Stein J, Vavricka S, European Crohn’s and Colitis Organisation [ECCO]. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohns Colitis. 2015;9(3):211–22.
NHS Digital. Hospital episode statistics (HES). Leeds, UK 2019. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics. Accessed Oct 22 2019.
Koch TA, Myers J, Goodnough LT. Intravenous iron therapy in patients with iron deficiency anemia: dosing considerations. Anemia. 2015;2015:763576.
Brainard J, Burmaster DE. Bivariate distributions for height and weight of men and women in the United States. Risk Anal. 1992;12(2):267–75.
Hermanussen M, Danker-Hopfe H, Weber GW. Body weight and the shape of the natural distribution of weight, in very large samples of German, Austrian and Norwegian conscripts. Int J Obes Relat Metab Disord. 2001;25(10):1550–3.
Radia D, Momoh I, Dillon R, Francis Y, Cameron L, Fagg TL, Overland H, Robinson S, Harrison CN. Anemia management: development of a rapid-access anemia and intravenous iron service. Risk Manag Healthc Policy. 2013;6:13–22.
Schmier J, Ogden K, Nickman N, Halpern MT, Cifaldi M, Ganguli A, Bao Y, Garg V. Costs of providing infusion therapy for rheumatoid arthritis in a hospital-based infusion center setting. Clin Ther. 2017;39(8):1600–17.
Afzali A, Ogden K, Friedman ML, Chao J, Wang A. Costs of providing infusion therapy for patients with inflammatory bowel disease in a hospital-based infusion center setting. J Med Econ. 2017;20(4):409–22.
Jones M, Maria H, Scott S, Wexler S (2019) The earlier, the better: a real-world experience of peri-operative anaemia management from Royal United Hospital, Bath, UK. Abstract P33. Presented at the 20th annual NATA symposium, April 4–5, 2019. Berlin, Germany.
Aksan A, Işık H, Radeke HH, Dignass A, Stein J. Systematic review with network meta-analysis: comparative efficacy and tolerability of different intravenous iron formulations for the treatment of iron deficiency anaemia in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2017;45(10):1303–18.
Aksan A, Işık H, Radeke HH, Dignass A, Stein J. Letter: the importance of dosing and baseline haemoglobin when establishing the relative efficacy of intravenous iron therapies-authors’ reply. Aliment Pharmacol Ther. 2017;46(7):705–6.
Pollock RF, Muduma G. A systematic literature review and indirect comparison of iron isomaltoside and ferric carboxymaltose in iron deficiency anemia after failure or intolerance of oral iron treatment. Expert Rev Hematol. 2019;12(2):129–36.
Frigstad SO, Haaber A, Bajor A, Fallingborg J, Hammarlund P, Bonderup OK, Blom H, Rannem T, Hellström PM. The NIMO scandinavian study: a prospective observational study of iron isomaltoside treatment in patients with iron deficiency. Gastroenterol Res Pract. 2017;2017:4585164.
Dahlerup JF, Jacobsen BA, van der Woude J, Bark LÅ, Thomsen LL, Lindgren S. High-dose fast infusion of parenteral iron isomaltoside is efficacious in inflammatory bowel disease patients with iron-deficiency anaemia without profound changes in phosphate or fibroblast growth factor 23. Scand J Gastroenterol. 2016;51(11):1332–8.
Wolf M, Rubin J, Achebe M, Econs M, Peacock M, Imel E, Thomsen L, Carpenter T, Weber T, Zoller H. Effects of iron isomaltoside versus ferric carboxymaltose on hormonal control of phosphate homeostasis: the PHOSPHARE-IDA04/05 randomized controlled trials. J Endocr Soc. 2019;3(1):OR13.
Acknowledgements
Funding
The development of the resource impact model, execution of the analyses, and preparation of the manuscript were funded by Pharmacosmos UK Limited, a wholly owned subsidiary of Pharmacosmos A/S. The Rapid Service and Open Access Fees were funded by Pharmacosmos UK Limited.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Frank Loughnane has nothing to disclose. Gorden Muduma is a full-time employee of Pharmacosmos A/S, the marketing authorization holder for Monover. Richard F. Pollock is a director and shareholder in Covalence Research Ltd, which received consultancy fees from Pharmacosmos UK Limited (a wholly-owned subsidiary of Pharmacosmos A/S) to develop the resource impact model, run the analyses, and write the manuscript.
Compliance with Ethics Guidelines
The analysis was based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to: https://doi.org/10.6084/m9.figshare.11637753.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Loughnane, F., Muduma, G. & Pollock, R.F. Development of a Resource Impact Model for Clinics Treating Pre-Operative Iron Deficiency Anemia in Ireland. Adv Ther 37, 1218–1232 (2020). https://doi.org/10.1007/s12325-020-01241-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-020-01241-0