Abstract
Introduction
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) are the standard of care for patients with EGFR mutation-positive non-small cell lung cancer (NSCLC). However, questions remain about the optimal treatment sequence of EGFR TKIs. The global, observational GioTag study demonstrated prolonged time on treatment with sequential afatinib and osimertinib therapy in patients who acquired the T790M mutation. Here, we assessed outcomes in patients who received the approved 40-mg starting dose of afatinib, as used in the clinical trial setting.
Methods
In the non-interventional, global, multicenter GioTag study, patients had T790M-positive disease following first-line afatinib and started osimertinib treatment ≥ 10 months prior to data entry. Primary outcome was time on treatment. This subanalysis assessed outcomes in patients who received afatinib 40 mg.
Results
In 169 patients who received an afatinib starting dose of 40 mg, median time on treatment was 27.6 months (90% confidence interval [CI] 26.3–31.3). Benefit was seen across patient subgroups, particularly those with Del19-positive disease and Asian patients; median time on treatment was 29.9 months (90% CI 27.6–46.7) in patients with Del19-positive disease and 46.7 months (90% CI 28.4–not reached) in Asian patients. The 2-year overall survival rate was 80%.
Conclusions
These real-world results support the overall study results and demonstrate prolonged time on treatment with sequential afatinib and osimertinib. The results suggest that sequential afatinib and osimertinib is a feasible therapeutic strategy for patients who acquire the T790M mutation, particularly those with Del19-positive disease or Asian patients.
Trial Registration Number
NCT03370770.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The optimal treatment sequence in patients with EGFR mutation-positive NSCLC is currently unknown. |
The non-interventional GioTag study demonstrated clinical benefit with sequential afatinib and osimertinib in patients with EGFR mutation-positive NSCLC with T790M-acquired resistance. |
This post hoc analysis aimed to determine the clinical benefit of sequential afatinib and osimertinib in patients who received the approved 40-mg starting dose, as used in the clinical trial setting. |
Our results further demonstrate prolonged clinical benefit with sequential afatinib and osimertinib therapy (median time on treatment of 27.6 months [90% CI 26.3–31.3]), and particular benefit for those with Del19-positive disease (29.9 months [90% CI 27.6–46.7]) and Asian patients (46.7 months [90% CI 28.4–not reached]). |
Together with findings from the overall study population, the results of the present analysis support sequential afatinib and osimertinib as a feasible therapeutic strategy. |
Introduction
Three generations of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) are commercially available for the treatment of patients with EGFR mutation-positive non-small cell lung cancer (NSCLC) [1]. Afatinib and dacomitinib (both second-generation irreversible ERBB family blockers), and osimertinib (a third-generation EGFR wild-type-sparing, irreversible EGFR/T790M inhibitor) demonstrated superior progression-free survival (PFS) versus the first-generation reversible EGFR TKIs erlotinib and gefitinib in head-to-head trials [2,3,4]. Furthermore, numerical improvements in overall survival (OS) were observed with second- and third- versus first-generation EGFR TKIs [3,4,5].
Regardless of the choice of first-line EGFR TKI, acquired resistance is inevitable, the predominant molecular resistance mechanism to gefitinib, erlotinib, and afatinib being the emergence of the “gatekeeper” T790M mutation (in ~ 50–70% of patients) [6,7,8,9]. This finding prompted development of the T790M-directed EGFR TKI osimertinib, which was first approved in the second-line setting on the basis of impressive efficacy following failure of first-line TKIs [8].
In contrast to first- and second-generation EGFR TKIs, treatment options following first-line osimertinib are less clear because of heterogeneous resistance mechanisms that are either not fully understood or not susceptible to currently available drugs [10, 11]. Furthermore, in an analysis of 91 patients treated with first-line osimertinib in the FLAURA study, no putative resistance mechanism was identified in > 60% of tumors analyzed [11]. Consequently, there is debate regarding whether osimertinib would be best reserved for second-line use, given that many patients treated with first- and second-generation EGFR TKIs up-front would be expected to be eligible to receive and benefit from subsequent osimertinib, thus prolonging the chemotherapy-free period [1, 12]. On the other hand, there is a risk in holding back osimertinib, which is now also approved as a first-line treatment option, having demonstrated superior efficacy and safety versus first-generation TKIs in this setting [4]. Indeed, not all patients treated with first- or second-generation EGFR TKIs will develop T790M-positive tumors and, thus, would not be eligible to receive osimertinib if it was not given as front-line treatment.
Further research is needed to determine the optimal treatment sequence in patients with EGFR mutation-positive NSCLC and, to date, few studies have assessed the cumulative benefit of sequential EGFR TKI regimens. To address this need, the non-interventional GioTag study was conducted in 204 patients with EGFR mutation-positive NSCLC with T790M-acquired resistance who were treated with sequential afatinib and osimertinib in the clinical practice setting. In the overall real-world population, the median time on treatment was 27.6 months across all patients, 46.7 months in Asian patients, 30.3 months in patients with Del19-positive tumors, and 36.4 months in those with both Del19-positive tumors and Eastern Cooperative Oncology Group performance status (ECOG PS) 0/1. Clinical benefit was observed across all patient subgroups, including those with poor prognostic factors such as ECOG PS ≥ 2 (median time on treatment 22.2 months) and brain metastases (median 19.4 months) [12]. Overall survival data were immature at the time of the initial analysis (June 2018; median follow-up 28.2 months) [12], but a subsequent interim analysis in 94 patients (April 2019; median follow-up 30.3 months) reported a median OS of 41.3 months [13]. While the approved afatinib starting dose is 40 mg, 17% of patients in GioTag received a modified starting dose of afatinib (most commonly 30 mg); hence, we conducted this post hoc analysis to determine the clinical benefit of sequential afatinib and osimertinib in patients who received the approved 40-mg starting dose, as used in the clinical trial setting.
Methods
Study Design and Patients
Full details of the GioTag study design have been published previously [12]. The global, observational, multicenter GioTag study (NCT03370770) was conducted across 10 countries (Austria, Canada, Israel, Italy, Japan, Singapore, Slovenia, Spain, Taiwan, and the USA) between December 2017 and May 2018. Medical and electronic health records of consecutive patients treated with sequential afatinib and osimertinib in real-world clinical practice were retrospectively reviewed. Eligible patients had EGFR mutation-positive (Del19/L858R) TKI-naïve advanced NSCLC, received first-line afatinib, and developed the T790M mutation, and subsequently started on second-line osimertinib (at least 10 months prior to data entry). All patients provided informed consent.
Endpoints and Assessments
The primary outcome was time on treatment, defined as the time from the first dose of afatinib to the last dose of osimertinib or death. Treatment interruptions and dose reductions for afatinib and osimertinib were documented. EGFR mutation and T790M status were determined according to local methodology and practice [12].
Statistical Analysis
Time on treatment and OS were estimated using Kaplan–Meier analysis, with time on treatment censored at data cutoff for patients still on treatment.
The present post hoc analysis uses data from the first, full analysis (database lock, June 2018). Baseline demographics, disease characteristics, osimertinib starting dose, time on treatment with sequential afatinib and osimertinib, time on treatment for each EGFR TKI, and OS were assessed in the subgroup of patients treated with a 40-mg starting dose of afatinib. Additionally, treatment duration analyses in this subgroup were further subdivided by ethnicity, age, EGFR mutation type, presence of brain metastases, and ECOG PS.
Compliance with Ethics Guidelines
The study was carried out in compliance with the protocol, the principles laid down in the Declaration of Helsinki, in accordance with the International Conference on Harmonisation Harmonized Tripartite Guideline for Good Clinical Practice, Good Epidemiological Practice, Guidelines for Good Pharmacoepidemiology Practice, and relevant sponsor standard operating procedures. The study was initiated only after all required legal documentation was reviewed and approved by the respective institutional review board/independent ethics committee (the Ethics Committee of the City of Vienna for the principal investigator MJH; all other ethics committees are listed in Supplementary Table 1) and competent authority according to national and international regulations.
Results
Patients
Of 204 patients included in the GioTag study, 169 received afatinib at the approved starting dose of 40 mg/day (40-mg starters). Baseline characteristics in these 40-mg starters were similar to those in the overall patient population (Table 1) [12], with patients in both groups being ethnically diverse; baseline characteristics were also similar in patients who received afatinib 40 mg and those who received < 40 mg (Supplementary Table 2). Patient enrollment by country for the 40-mg starters is shown in Supplementary Table 3; patient baseline demographic and disease characteristics for Asian and non-Asian patients are shown in Supplementary Table 4. At the start of afatinib treatment, 123 (72.8%) patients in the 40-mg subgroup had a Del19 mutation and 45 (26.6%) had the L858R mutation (one patient had both Del19 and L858R mutations). At the start of osimertinib treatment, all patients had a documented T790M mutation. Consistent with the overall population, 17 (10.1%) patients had brain metastases at the start of afatinib treatment. ECOG PS was 0, 1, or ≥ 2 in 39 (23.2%), 87 (51.8%), and 28 (16.7%) patients, respectively, at the start of afatinib treatment. As seen for the overall population, the proportion of patients with higher ECOG PS was greater at the start of osimertinib treatment (data not shown).
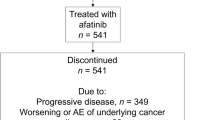
The main reason for discontinuation of afatinib (n = 159/169 [94.1%]) and osimertinib (n = 79/87 [74.5%]) was progressive disease (Supplementary Fig. 1). At the time of database lock (June 29, 2018), 87 [51.5%) patients had discontinued osimertinib and 82 remained on treatment.
Time on Treatment
With median follow-up of 28.2 months (range 14.0–96.8 months), the median time on treatment for sequential afatinib and osimertinib in the afatinib 40-mg starters was 27.6 months (90% CI 26.3–31.3; Fig. 1). Median time on afatinib was 11.5 months (90% CI 10.5–12.2) and median time on osimertinib was 14.6 months (90% CI 13.2–15.9). The median time between stopping afatinib and starting osimertinib treatment was 0.6 months (90% CI 0.5–0.7).
Overall time on treatment, and time on afatinib and osimertinib according to patient subgroups (by ethnicity, EGFR mutation type, presence of brain metastases, ECOG PS, and age) are shown in Table 2 and Fig. 2. Clinical benefit with sequential afatinib and osimertinib was observed across all patient subgroups, with a particularly prolonged median time on treatment reported for Asian patients (overall 46.7 months; afatinib 14.0 months; osimertinib 19.6 months). Median times on sequential and individual EGFR TKI treatments were numerically longer in patients with Del19 versus L858R mutations (overall 29.9 vs 18.8 months; afatinib 12.2 vs 9.4 months; osimertinib 15.0 vs 9.4 months) and in patients with ECOG PS 0/1 vs ≥ 2 (overall 31.3 vs 20.9 months; afatinib 12.0 vs 10.0 months; osimertinib 15.9 vs 8.4 months). Patient age at start of therapy did not affect time on treatment (median 28.1 months for patients aged < 65 years and 27.6 months for those aged ≥ 65 years), while median time on treatment was numerically longer in patients without versus those with baseline brain metastases (overall 27.6 vs 22.2 months; afatinib 11.6 vs 10.5 months; osimertinib 14.6 vs 10.2 months).
Overall time on treatment with sequential afatinib and osimertinib by subgroup: i ethnicity; ii type of activating EGFR mutation at baseline; iii presence or absence of brain metastases at baseline; iv ECOG PS at baseline; v age (< 65 vs ≥ 65 years). ECOG PS Eastern Cooperative Oncology Group performance status
Overall Survival
OS data for the 40-mg starters were immature at the time of database lock. OS analysis among patients who started on 40 mg was therefore limited to landmarks of 2 and 2.5 years, where maturity was 45.3% and 34.5%, respectively. The 2-year and 2.5-year OS rates from the start of afatinib treatment were 80% and 70%, respectively.
Discussion
This post hoc analysis of the non-interventional, global GioTag study provides further evidence for prolonged clinical benefit with sequential afatinib and osimertinib therapy, with results in patients who received the approved 40-mg starting dose of afatinib consistent with those previously reported for the overall patient population [12]. This is an important finding as, without evidence, it cannot automatically be assumed that the overall study results are applicable to a patient subgroup. Further, with questions remaining about the optimum treatment strategy for EGFR mutation-positive NSCLC, these results support sequential afatinib followed by osimertinib as a feasible therapeutic option in patients who acquire the T790M mutation.
Consistent with the overall GioTag study population, the 169 patients who received a 40-mg starting dose of afatinib were of diverse ethnicity, and there were no notable differences in the proportion of patients with ECOG PS 2/3 or type of EGFR mutation (Del19/L858R) when compared to the overall patient population [12]. The median time on treatment with sequential afatinib and osimertinib in patients receiving a starting dose of 40 mg afatinib (27.6 months [90% CI 26.3–31.3]) was also consistent with that seen for the overall GioTag population (27.6 months [90% CI 25.9–31.3]), as were the median times for individual afatinib (11.5 months) and osimertinib (14.6 months) treatments (11.9 months and 14.3 months, respectively, in the overall population). It should be noted that the median overall time on treatment, time on afatinib, and time on osimertinib may be from three different patients; hence, the median overall time on treatment would not be expected to equal the sum of the median times on afatinib and osimertinib. Importantly, these findings were consistent across patient subgroups, with particular benefit seen in Asian patients and those with Del19-positive disease.
The particular benefit in patients with Del19-positive disease versus those with L858R-positive disease was also seen in the overall real-world patient population [12, 13], and in the phase III LUX-Lung 3 and 6 studies [14]. Indeed, it has been suggested that Del19- and L858R-positive tumors be considered as separate disease entities [13]. The clinical benefit with sequential afatinib and osimertinib treatment in Del19-positive patients is particularly relevant considering that Del19-positive tumors are more likely to acquire the T790M mutation (75% likelihood compared with up to 58% for L858R-positive tumors) [9, 15, 16]. Further, and importantly for real-world clinical practice, patients with characteristics associated with a poor prognosis, such as those with ECOG PS ≥ 2 and stable brain metastases, also derived clinical benefit with the approved 40-mg starting dose of afatinib used in clinical trials.
The main limitations of this analysis relate to the retrospective nature of the GioTag study and potential for selection bias. However, to minimize the potential for selection bias, the study only included consecutive patients who fulfilled all of the inclusion criteria and enrollment was limited to a maximum of 15 patients per site. The other main limitation of the study was the lack of a comparator arm, which limits interpretation of the results.
Together with the overall study results [12], the results of this post hoc analysis provide further evidence for the effectiveness of sequential treatment with a starting dose of 40 mg afatinib followed by osimertinib in patients with EGFR mutation-positive NSCLC who acquire T790M. While there is strong evidence for the use of osimertinib in the front-line setting [4], given the lack of targeted therapeutic options following osimertinib therapy [10, 11], the data reported here suggest that sequential afatinib and osimertinib may effectively prolong the chemotherapy-free treatment period.
Conclusions
The results of this subgroup analysis are consistent with the overall findings from the GioTag study, demonstrating prolonged time on treatment with sequential afatinib and osimertinib in the subgroup of patients who received a 40-mg afatinib starting dose. Together with previous findings for the overall study population [12], the results of the present analysis support sequential afatinib and osimertinib as a feasible therapeutic strategy, resulting in a median chemotherapy-free treatment time of 27.6 months for both the overall patient population—treated in the real-world setting—and patients who received a 40-mg starting dose of afatinib, a subgroup which more closely reflects the clinical trial setting.
References
Girard N. Optimizing outcomes in EGFR mutation-positive NSCLC: which tyrosine kinase inhibitor and when? Future Oncol. 2018;14(11):1117–32.
Park K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016;17(5):577–89.
Wu YL, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(11):1454–66.
Soria JC, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–25.
Paz-Ares L, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol. 2017;28(2):270–7.
Arcila ME, et al. Rebiopsy of lung cancer patients with acquired resistance to EGFR inhibitors and enhanced detection of the T790M mutation using a locked nucleic acid-based assay. Clin Cancer Res. 2011;17(5):1169–80.
Sequist LV, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3(75):75ra26.
Yang JC, et al. Osimertinib in pretreated T790M-positive advanced non-small-cell lung cancer: AURA study phase II extension component. J Clin Oncol. 2017;35(12):1288–96.
Hochmair MJ, et al. Liquid-biopsy-based identification of EGFR T790M mutation-mediated resistance to afatinib treatment in patients with advanced EGFR mutation-positive NSCLC, and subsequent response to osimertinib. Target Oncol. 2019;14(1):75–83.
Oxnard GR, et al. Assessment of resistance mechanisms and clinical implications in patients with EGFR T790M-positive lung cancer and acquired resistance to osimertinib. JAMA Oncol. 2018;4(11):1527–34.
Ramalingam SS, et al. Mechanisms of acquired resistance to first-line osimertinib: Preliminary data from the phase III FLAURA study. Ann Oncol. 2018;29(suppl_8):LBA50.
Hochmair MJ, et al. Sequential treatment with afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: an observational study. Future Oncol. 2018;14(27):2861–74.
Hochmair MJ, et al. Sequential afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: updated analysis of the observational GioTag study. Future Oncol. 2019;15(25):2905–14.
Yang JC, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16(2):141–51.
Jenkins S, et al. EGFR mutation analysis for prospective patient selection in two phase II registration studies of osimertinib. J Thorac Oncol. 2017;12(8):1247–56.
Ke EE, et al. A higher proportion of the EGFR T790M mutation may contribute to the better survival of patients with exon 19 deletions compared with those with L858R. J Thorac Oncol. 2017;12(9):1368–75.
Acknowledgements
We thank the patients, their families, the investigators and staff who participated in the study.
Funding
This study was supported by Boehringer Ingelheim, who also provided funding for Advances in Therapy’s Rapid Service Fee and Open Access charges.
Medical Writing and Additional Assistance
This work was supported by Boehringer Ingelheim. Contract Research Organization support was executed by Parexel. Medical writing assistance, supported financially by Boehringer Ingelheim, was provided by Jane Saunders of GeoMed, an Ashfield Company, part of UDG Healthcare plc, during the preparation of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Nobuyuki Yamamoto reports honoraria and research funding from Boehringer Ingelheim. Takeshi Mera is an employee of Boehringer Ingelheim. Angela Märten is an employee of Boehringer Ingelheim. Maximilian J. Hochmair reports honoraria from AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Merck Sharp & Dohme, Pfizer, and Roche, and has had consulting or advisory roles with Boehringer Ingelheim, Merck Sharp & Dohme, Novartis and Roche.
Compliance with Ethics Guidelines
The study was carried out in compliance with the protocol, the principles laid down in the Declaration of Helsinki, in accordance with the International Conference on Harmonisation Harmonized Tripartite Guideline for Good Clinical Practice, Good Epidemiological Practice, Guidelines for Good Pharmacoepidemiology Practice, and relevant sponsor standard operating procedures. The study was initiated only after all required legal documentation was reviewed and approved by the respective institutional review board/independent ethics committee (the Ethics Committee of the City of Vienna for the principal investigator MJH; all other ethics committees are listed in Supplementary Table 1) and competent authority according to national and international regulations.
Data Availability
The datasets generated and analyzed during the study are available from TM on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.11295440.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Yamamoto, N., Mera, T., Märten, A. et al. Observational Study of Sequential Afatinib and Osimertinib in EGFR Mutation-Positive NSCLC: Patients Treated with a 40-mg Starting Dose of Afatinib. Adv Ther 37, 759–769 (2020). https://doi.org/10.1007/s12325-019-01187-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-019-01187-y