Abstract
Introduction
Treatment of neovascular age-related macular degeneration (nAMD) has evolved with the advent of anti-vascular endothelial growth factor agents such that intravitreally administered aflibercept and ranibizumab (RBZ) have become the standard of care. Randomized clinical trials (RCTs) have demonstrated the benefits of these agents in nAMD; however, results achieved under RCT protocols may not always be replicated in clinical practice. Assessing real-world outcomes is important to estimate the effectiveness and cost-effectiveness of these two agents. Our objective was to assess the real-world effectiveness of intravitreally administered aflibercept and RBZ in treatment-naive patients with nAMD and determine the cost-effectiveness of intravitreally administered aflibercept versus RBZ in a real-world setting.
Methods
A multistage approach was undertaken. A systematic literature review (SLR) was completed to identify studies describing real-world outcomes in patients with nAMD treated intravitreally with aflibercept or RBZ. A meta-analysis of data identified in the SLR generated a pooled estimate of the effectiveness of intravitreally administered aflibercept and RBZ at 52 weeks and an estimate of treatment burden (injection frequency and monitoring). The impact of treatment effect modifiers, such as baseline visual acuity (VA) and age, were corrected through a multivariable meta-regression. A Markov state transition model was developed to estimate the real-world cost-effectiveness of intravitreally administered aflibercept using results from the pooled estimates for effectiveness and treatment burden as primary inputs. The analysis considered the perspective of the French National Healthcare System.
Results
Patients treated intravitreally with aflibercept had a mean age of 79.52 years and mean baseline VA of 55.80 Early Treatment Diabetic Retinopathy Study (ETDRS) letters. At week 52, mean VA gain was 5.30 ETDRS letters in patients reporting an average of 7.10 intravitreal injections of aflibercept and 8.65 visits (injection and/or monitoring). RBZ-treated patients were younger (77.28 years), with a lower mean baseline VA (52.81 ETDRS letters). At week 52, mean VA gain from baseline was 4.24 ETDRS letters, with an average of 5.88 injections and 10.10 visits (injection and/or monitoring). After correcting for differences in age (77.28 years) and baseline VA (52.81 ETDRS letters) and considering the current clinical practice with aflibercept and RBZ, the mean VA gain was 6.57 ETDRS letters for patients treated intravitreally with aflibercept and 4.42 ETDRS for patients treated intravitreally with RBZ. The cost-effectiveness analysis showed that intravitreally administered aflibercept is a more effective treatment option with an incremental gain in quality-adjusted life years (QALYs) (4.918 versus 4.880) and an incremental cost-effectiveness ratio (ICER) of €27,087 per QALY.
Conclusions
The analysis identified differences in the overall treatment approach and how ophthalmologists use intravitreally administered aflibercept and RBZ in clinical practice. These differences ultimately influence the mean real-world effectiveness of the two agents. Intravitreal treatment with aflibercept (injection frequency and patients follow-up) was consistent and in line with the European label recommendations. Patients treated intravitreally with aflibercept in clinical practice reported a mean gain in VA of similar magnitude to the mean VA gain reported in the pivotal RCT. Conversely, treatment with RBZ varied significantly across the different studies. On average, RBZ-treated patients reported a low injection frequency and a frequent follow-up, driven in part by the high number of patients treated with pro re nata (PRN) regimens. RBZ-treated patients reported gains in VA versus baseline; however, the magnitude of the gain in VA was not comparable to the VA gains reported in the RBZ pivotal RCT. Intravitreal treatment with aflibercept was associated with better mean VA outcomes and an incremental gain in QALYs compared with RBZ and can be considered cost-effective for the treatment of nAMD in patients in France despite a higher price for each individual intravitreal injection of aflibercept compared with RBZ.
Funding
Bayer AG, Basel.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
What is already known about this subject? |
Anti-vascular endothelial growth factor agents such as intravitreally administered aflibercept and ranibizumab (RBZ) have been shown to be efficacious in the treatment of neovascular age-related macular degeneration (nAMD) in the clinical trial setting, but results may not always be replicated in the real-world setting. |
Cost-effectiveness of intravitreally administered aflibercept and RBZ has largely been based on randomized clinical trials (RCTs), and to date, the real-world cost-effectiveness of these two treatments has not been analyzed. |
What was learned from the study? |
This study incorporated a systematic literature review, a meta-analysis, and a multivariable meta-regression to assess real-world effectiveness and overall treatment burden with intravitreally administered aflibercept and RBZ. |
The analysis identified differences in the overall treatment approach and how ophthalmologists use intravitreally administered aflibercept and RBZ in clinical practice. These differences ultimately influence the mean real-world effectiveness of the two agents. |
Patients with nAMD who were treated intravitreally with aflibercept experienced higher mean visual gains than those treated with RBZ, and the number of injections required to achieve clinically meaningful improvements in vision was lower with intravitreally administered aflibercept than with RBZ. |
From the perspective of the French healthcare system, intravitreally administered aflibercept was a cost-effective treatment option for nAMD despite a higher cost per individual injection. |
Introduction
Exudative or neovascular age-related macular degeneration (nAMD) is the most severe form of AMD and the most common cause of vision loss in the elderly population in the developed world [1]. Treatment of nAMD has changed dramatically with the advent of anti-vascular endothelial growth factors (VEGF) agents. Since the approval of ranibizumab (RBZ) and intravitreally administered aflibercept for treatment of nAMD in Europe in 2007 [2] and 2012 [3], respectively, anti-VEGF agents have become the standard of care. The pivotal randomized clinical trials (RCTs) ANCHOR and MARINA demonstrated the benefits of RBZ 0.5 mg intravitreal injection every 4 weeks in patients with nAMD [4, 5]. Subsequently, the VIEW studies demonstrated that intravitreally administered aflibercept 2 mg every 8 weeks (after three initial monthly injections) was noninferior to RBZ 0.5 mg every 4 weeks in all clinical endpoints at 1 year with lower injection frequency [6, 7]. The anti-VEGF clinical research programs were robust and proved the efficacy of these agents, but results achieved under strict protocols in RCTs may not always be replicated in routine clinical practice. Early real-world studies such as AURA found that RBZ-treated patients received fewer injections than those in RCTs, and patients did not report sustained visual acuity (VA) improvement [8]. AURA also showed that several factors, including age, baseline VA score, number of injections, and regular monitoring, may be predictive of treatment outcomes and may explain the differences between real-world outcomes and RCTs [9]. Since these studies were undertaken, clinical practice has evolved; recent meta-analyses of RBZ real-world studies [10, 11] showed that VA outcomes at 12 months in RBZ-treated patients were improved compared with those in AURA. Despite these improvements, treatment outcomes varied between studies, and the mean VA gain was lower in observational studies than in RCTs. These improvements were not maintained long term, particularly with treatment regimens that had low injection frequency [10]. A pro re nata (PRN) treatment strategy was widely adopted for RBZ-treated patients, leading to high variability in the number and frequency of injections.
Because of its later approval, there are fewer observational studies evaluating intravitreally administered aflibercept in patients with nAMD; however, these studies indicate that the implementation of a proactive treatment regimen with intravitreally administered aflibercept and an injection frequency consistent with the approved label are associated with RCT-like outcomes in clinical practice [12,13,14,15,16]. In a recent meta-analysis of studies on intravitreally administered aflibercept, researchers included subgroup analyses describing outcomes in RCTs and observational studies [17]. The mean VA gain at 12 months in the observational studies was lower but comparable to the gain observed in RCTs. The analysis also suggested that VA improvements were slightly higher with regular dosing (> 7 injections/year) than with irregular dosing.
The cost-effectiveness of intravitreally administered aflibercept and RBZ to treat patients with nAMD in the European setting was established primarily on the basis of data from RCTs [18,19,20,21,22]. Both intravitreally administered aflibercept and RBZ have been shown to be cost-effective treatment options in different settings, but no analysis of the real-world cost-effectiveness of these two treatments has been undertaken. As the published evidence shows, there are differences between the efficacy observed in RCTs compared with observational studies, so it is important to assess if these differences impact current cost-effectiveness assumptions.
We assessed the effectiveness of intravitreally administered aflibercept and RBZ in treatment-naive patients with nAMD and determined the cost-effectiveness of intravitreally administered aflibercept versus RBZ in a real-world setting.
Methods
A multistage approach, including a systematic literature review (SLR), a meta-analysis, and a meta-regression, was used to determine the effectiveness and treatment burden associated with the use of intravitreally administered aflibercept and RBZ in treatment-naive patients with nAMD in a real-world setting. To assess the real-world cost-effectiveness, a Markov decision model was developed and populated with the pooled estimates for treatment effectiveness and treatment burden derived from the meta-analysis. The model was adapted to reflect the perspective of the French healthcare system.
Systematic Literature Review
An SLR targeting prospective observational studies including treatment-naive patients with nAMD receiving intravitreally administered aflibercept or RBZ that reported data for 1 year of follow-up was conducted. The SLR included a Medline database search via ProQuest and a search of congress proceedings for the Association for Research in Vision and Ophthalmology (2014–2017), American Academy of Ophthalmology (2014–2016), European Society of Retina Specialists (2014–2017), World Ophthalmology Congress (2014, 2016, 2018) and Asia–Pacific Academy of Ophthalmology (2014–2017) for the years indicated. The SLR included studies published through July 24, 2017. Further details are included in Appendix 1 in the supplementary material.
Previous meta-analyses of anti-VEGF agents have included both prospective and retrospective studies to derive pooled estimates for VA change. Inclusion of mixed design studies can increase the bias of the analysis by increasing imprecision, incomplete follow-up, and recall bias normally associated with retrospective studies. To overcome this limitation and reduce the associated bias, this meta-analysis focused on prospective observational studies or data collected prospectively. To reduce publication bias and uncertainty associated with small sample studies, publications reporting data from studies that enrolled fewer than 40 patients were also excluded.
All search hits were imported into Excel, and data from eligible abstracts and full-text studies were extracted and selected on the basis of relevant populations, interventions, comparators, outcomes, and study design [23]. The data extraction template was built using the Meta-Analyses and Systematic Reviews of Observational Studies guidelines [24], and the reporting of the SLR followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [23]. Screening of title, abstract, and full text was performed by one reviewer using PRISMA guidelines [23]. Inclusion and exclusion criteria are summarized in Appendix 1 in the supplementary material.
Meta-Analysis
A meta-analysis of the data identified from the SLR was undertaken. Inclusion criteria for the meta-analysis were studies of treatment-naive patients with nAMD who received intravitreal treatment with aflibercept or RBZ, reported 52-week effectiveness outcomes, and included at least 40 patients per study. The primary objective of the meta-analysis was to derive a pooled estimate for the variables of patient characteristics (age and baseline VA), treatment burden (defined as mean number of injections and injection/monitoring visits from baseline to week 52), and effectiveness (defined as VA change from baseline to week 52 in Early Treatment Diabetic Retinopathy Study (ETDRS) letters). These variables were identified as key parameters in a previous meta-analysis of real-world outcomes with anti-VEGF therapy [10]. When studies did not report the standard deviation (SD) necessary for the meta-analysis, these values were imputed via the Multivariate Imputation by Chained Equations (MICE) algorithm [25]. When necessary, variables of interest were converted: number of eyes to number of patients, subgroup mean and SD to aggregate mean and SD, logMAR and decimal to ETDRS letters, SDlogMAR to SDletters, P value to SD [via T value and standard error (SE)], confidence interval (CI) to SD [via standard error (SE)], and SE to SD. Fixed-effects and random-effects models were used to produce estimates for all dependent variables. Heterogeneity was assessed using the I2 statistic [26], and model selection was based on the Bayesian information criterion (BIC). Meta-analyses were performed using the Metafor R package [27] and the MICE algorithm in R [25] (for multiple imputations). Outcomes are reported as means (95% CI).
Multivariable Meta-Regression
A multivariable meta-regression analysis was undertaken to correct for differences in patients’ baseline characteristics. Exploratory covariates included in the meta-regression were age at baseline, baseline VA, number of injections, and treatment (intravitreally administered aflibercept or RBZ). The outcome variable assessed was mean VA change (ETDRS letters) at 52 weeks. In the meta-regression, VA change was modelled using the following equation:
A model without the interaction between the number of injections and treatment was considered, but the BIC preferred the model with the interaction term.
Cost-Effectiveness Model
Model Structure and Perspective
A Markov state transition model was developed to assess the cost-effectiveness of intravitreally administered aflibercept. The Markov model was structured so health states were distributed on the basis of patients’ vision using average VA score (ETDRS letters) at a series of inflection points, including baseline, 12 weeks (treatment efficacy), 1 year (post-treatment efficacy), and 15 years (long-term decline). Because overall vision and relationship with quality of life are functions of vision in both eyes, model health states were defined by patients’ best-corrected VA score of both eyes. At any point in the model, patients are distributed into one of eight mutually exclusive, exhaustive VA health states, defined by ETDRS letters (graphical representation of the model is available in Appendix 2 in the supplementary material). The distribution of patients across each VA health state was estimated using the mean and SD of VA at each point in time and assuming a scaled beta distribution (bound at 0 and 100, as per the ETDRS letter scale). The model allows for selecting various time horizons from 10 years to lifetime, thus providing an adequate time frame to capture all relevant treatment costs and effects [measured as quality-adjusted life years (QALYs)] associated with the interventions. To ensure that the analysis provided a complete overview, various time horizons were considered. The analysis considers recommendations from the French National Healthcare System, Haute Autoritié de Santé (HAS), and includes both direct and indirect costs [28]. Costs and outcomes were discounted at a 4% annual rate per HAS guidelines.
Compliance with Ethics Guidelines
This study is based on previously conducted studies and does not involve any new studies of human participants or animal subjects performed by any of the authors. This study did not require ethical approval as it did not involve human participants or animal subjects.
Model Inputs
Treatment effectiveness inputs used in the model were derived primarily from the meta-analysis and meta-regression, except for effectiveness at week 12, because the meta-analysis did not provide a pooled estimate for this time point. To overcome this limitation, the data were derived from two real-world studies of intravitreally administered aflibercept (RAINBOW [12]) and RBZ (ORACLE [29]). The impact of this assumption was tested in a sensitivity analysis. Long-term decline was based on assessment of VA decline in an elderly UK population, which estimated a decline of 0.96 ETDRS letters per year [30]. The model considered the incidence of adverse events in the VIEW trials [6], given that evidence suggests that real-world incidence is in line with trial data. The duration of treatment was assumed to be 5 years, as in previous analyses. Because the meta-analysis did not provide resource use beyond 52 weeks, injection frequency data for this model were derived from two French studies, Duval et al. [31] for intravitreally administered aflibercept (assumed constant use for years 2–5) and Boulanger-Scemama et al. [32] for RBZ.
Base-case utilities were derived from published literature. Czoski-Murray used contact lenses to simulate the effects of visual impairment caused by nAMD and estimated a linear relationship between best-seeing eye VA (logMAR) and time trade-off (TTO) utility [33]. This was used to estimate utility values for model VA health states using the following equation:
Estimates of costs were derived from published sources and grouped into six categories: (1) treatment; (2) administration of treatment; (3) monitoring and follow-up; (4) treating adverse events; (5) health transport; and (6) blindness costs. Cost analysis details are shown in Appendix 2 in the supplementary material.
Results
Systematic Literature Review
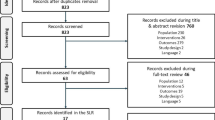
The results of the SLR search are shown in Fig. 1. Seventy-two studies (56 full-text publications and 16 conference abstracts) met the inclusion and exclusion criteria. Of the studies identified, some reported subgroup data that were considered as separate observations for the meta-analysis. After assessment, it was determined that the 72 publications provided data for 108 observations.
Meta-Analysis
The 72 publications identified in the SLR were additionally screened to assess if the publication reported data on the minimum set of variables necessary to conduct the meta-analysis. The publications (observations) had to describe studies conducted in treatment-naive populations, report data on injection frequency and treatment outcomes (VA change from baseline) at week 52, include at least 40 patients and, in the case of abstracts, be published after 2014. Additionally, publications were screened to identify and remove duplicates. If two or more publications reported data from the same source/population, only one publication was selected and included in the meta-analysis. Publications reporting data on more variables or in larger populations were preferred (Fig. 1). After review, 24 observations from 23 studies were selected for inclusion in the meta-analysis (7 observations for intravitreally administered aflibercept [12,13,14,15,16, 34, 35] and 17 for RBZ [34, 36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51]) (Appendix 1 in the supplementary material). One study contributed more than one observation because it reported several subgroups, including both intravitreally administered aflibercept and RBZ [34].
The analyses showed strong between-study heterogeneity (quantified by I2), so the random-effects model was preferred over the fixed-effects model. Patients treated intravitreally with aflibercept had a mean age of 79.52 years and a mean baseline VA of 55.80 ETDRS letters. At week 52, patients received on average 7.10 injections and reported 8.65 visits (overall treatment burden including injection and/or monitoring). The mean VA gain at 52 weeks versus baseline was 5.30 ETDRS letters (Table 1; Fig. 2a) with low between-study heterogeneity. Compared with patients treated intravitreally with aflibercept, RBZ-treated patients were younger (77.28 years) and had a lower mean baseline VA (52.81 ETDRS letters). At week 52, RBZ-treated patients received on average 5.88 injections and reported 10.10 visits (injection and/or monitoring). The mean VA gain at 52 weeks versus baseline was 4.24 ETDRS letters (Table 1; Fig. 2b).
Multivariable Meta-Regression
Eighteen observations were included in the meta-regression (5 for intravitreally administered aflibercept [12,13,14,15, 52] and 13 for RBZ [36,37,38,39,40,41, 43,44,45,46,47,48, 51]; Appendix 1 in the supplementary material). In the multivariable meta-regression, higher age and higher baseline VA were associated with lower VA improvements, while intravitreally administered aflibercept was associated with higher VA gains compared with RBZ (Appendix 1, Table G in the supplementary material). Injection frequency showed positive association with VA change, while the interaction term between the number of injections and aflibercept was negative. These findings suggest that the VA gains become larger with a greater number of injections for both RBZ and intravitreally administered aflibercept. This is consistent with findings from a previous meta-analysis of anti-VEGF agents in which poorer outcomes with RBZ were associated with lower injection frequency in clinical practice compared with RCTs [10]. With low numbers of injections, intravitreally administered aflibercept is associated with larger gains than RBZ, but this advantage diminishes with higher numbers of injections because the marginal gains with an additional injection are greater for RBZ. The above findings are subject to sampling errors; the coefficients of age, baseline VA, intravitreally administered aflibercept, and the interaction term were statistically insignificant. The multivariable meta-regression included only studies that reported SDs. Sensitivity analyses including studies with imputed SDs showed comparable results.
To determine the effectiveness of treatment (intravitreally administered aflibercept/RBZ) on VA gains, while considering the difference in the number of injections in the real world, it was assumed that patients treated intravitreally with aflibercept and RBZ had similar baseline characteristics. Baseline values were defined on the basis of pooled data from the RBZ group (mean age 77.28 years; mean baseline VA 52.81 ETDRS letters). After correcting for age and baseline VA, the mean VA gain for patients treated intravitreally with aflibercept was 6.57 ETDRS letters, with patients receiving 7.10 intravitreal injections in the first 52 weeks. The mean VA gain for RBZ-treated patients was 4.42 ETDRS, with patients receiving 5.88 injections in the first 52 weeks.
Using the meta-regression results, it was possible to calculate the impact of injection frequency and treatment (intravitreally administered aflibercept/RBZ) on VA gain and determine the resources needed to achieve clinically meaningful outcomes. Our analysis showed that patients treated intravitreally with aflibercept can achieve a clinically meaningful improvement in VA of 5 ETDRS letters (1 line of vision) with 5–6 injections, whereas RBZ-treated patients would require 6–7 injections to surpass the 5-letter gain versus baseline.
Cost-Effectiveness
The base case assumed a patient population (mean age, 77.28 years; baseline VA, 52.81 ETDRS letters) receiving active treatment for 5 years and assessed cost-effectiveness for a 15-year time horizon. Intravitreal treatment with aflibercept was associated with an incremental gain in effectiveness compared with RBZ (4.918 versus 4.880 QALYs, respectively), although at a higher overall treatment cost (€18,187 versus €17,168, respectively). The incremental cost-effectiveness ratio (ICER) of intravitreally administered aflibercept versus RBZ was €27,087 per QALY (Table 2). For a 10-year time horizon, the estimated mean incremental QALYs for intravitreally administered aflibercept versus RBZ was + 0.036, and the mean incremental cost was + €1022, resulting in an ICER of €28,185 per QALY. Assuming a lifetime horizon, the mean incremental QALYs for intravitreally administered aflibercept versus RBZ was + 0.037 with a mean incremental cost of + €1023, resulting in an ICER of €28,185 per QALY.
Cost-Effectiveness Sensitivity Analysis
To assess the impact of the assumptions made for effectiveness of intravitreally administered aflibercept and RBZ at week 12, a sensitivity analysis was completed assuming similar effectiveness for both agents. In this scenario, the ICER was similar (€29,687/QALY to €29,694/QALY). The probabilistic sensitivity analysis estimated that the mean incremental cost was + €690 and the mean incremental QALYs was + 0.044, with a mean ICER of €15,620 per QALY (Appendix 2 in the supplementary material). A cost-effectiveness acceptability curve indicated that for a threshold of €30,000 per QALY, there was a 70% probability of intravitreally administered aflibercept being cost-effective versus RBZ (Appendix 2 in the supplementary material).
Discussion
This analysis provides a comprehensive assessment of both the effectiveness and cost-effectiveness of intravitreally administered aflibercept and RBZ in treatment-naive patients with nAMD in a real-world setting.
In our analysis, patients treated intravitreally with aflibercept reported a mean baseline age of 79.52 years and VA of 55.80 ETDRS letters and achieved a mean VA gain of 5.30 ETDRS letters at 52 weeks; these patients received on average 7.10 injections and reported 8.65 visits (injection and/or monitoring). Conversely, RBZ-treated patients had a lower mean baseline age of 77.28 years and VA score of 52.81 ETDRS letters and achieved a mean gain of 4.24 ETDRS letters with 5.88 injections and 10.10 visits (injection and/or monitoring) at 52 weeks. After correcting for differences in age and baseline VA to those of the RBZ population, while upholding the current clinical practice, the mean gain in VA at week 52 was 6.57 ETDRS letters for patients treated intravitreally with aflibercept and 4.42 ETDRS letters for RBZ-treated patients. With current clinical practice, patients treated intravitreally with aflibercept experience a mean gain from baseline of more than 1 line of vision (5 ETDRS letters), and the mean number of injections and visits is consistent with the European prescribing information for nAMD (i.e., three initial doses every 4 weeks followed by dosing every 8 weeks over the first 52 weeks). Pooled estimates indicate that real-world outcomes with intravitreally administered aflibercept are consistent across studies and populations. The analysis found that RBZ-treated patients achieved a mean VA gain of 4.42 ETDRS letters with 5.88 injections and 10.10 visits (injection and/or monitoring). The mean injection frequency observed in clinical practice varied significantly among the RBZ studies analyzed, with many of the publications reporting a lower number of injections than the pivotal RCTs. PRN dosing is still used in the real-world setting, which may lead to fewer injections than are needed, potentially resulting in suboptimal outcomes. The findings of our RBZ analysis align with the findings from Kim et al. [10] who concluded that the mean overall gain in RBZ-treated patients was 5 ETDRS letters. The mean gain in VA observed in clinical practice is lower than the mean gain reported in the pivotal trials. Kim et al. [10] include subanalysis of the RBZ real-world studies assessing separately the outcomes registered with PRN regimens and treat and extend (T&E) regimens. Patients treated following a T&E regimen received more injections than patients treated following a PRN regimen and reported better outcomes at week 52. The T&E regimens with RBZ were associated with better outcomes and a reduction in the overall treatment burden for the patient. These findings suggest that the poorer outcomes achieved in a real-world setting could be linked to lower injection frequency (the pooled analysis indicated that the mean number of injections was significantly below that reported in the pivotal trials).
The efficacy and safety of intravitreally administered aflibercept and RBZ are well established and both drugs proved to be efficacious and safe when used according to the label recommendations [2, 3]. Our analysis, like that of Kim et al. [10], describes a scenario were RBZ PRN regimens are still frequently used to manage patients and are associated with poorer outcomes. Two of the studies included in this analysis, Finger et al. [43] and Wickremasinghe et al. [51], generate data describing the benefits of using the RBZ T&E in patients with nAMD. The positive impact on patient outcomes and treatment burden associated with T&E should be considered.
In our analysis we explored the relationship between injection number and VA changes, and our findings reinforced the assumption that an injection frequency similar to that reported in RCTs can contribute to the achievement of RCT-like VA improvements for patients with nAMD; these findings are valid for patients treated intravitreally with aflibercept and RBZ. Our analysis also suggests that patients treated intravitreally with aflibercept can achieve a clinically meaningful improvement in VA [i.e., gain of 5 ETDRS letters (1 line of vision)] with 5–6 injections. However, to achieve a similar outcome, RBZ-treated patients would require 6–7 injections. In our analysis we corrected for treatment effect modifiers such as age and baseline characteristics, but further research is needed to determine unequivocally the relationship between injection frequency and outcomes.
Overall, our findings suggest that, with the current clinical practice, treatment-naive patients with nAMD treated intravitreally with aflibercept achieve higher mean gains in VA than those treated with RBZ. The analysis shows that the treatment approach with intravitreally administered aflibercept is consistent with the European label recommendations [3], suggesting that a proactive regimen with an injection frequency in line with the label contributed to delivering positive outcomes that are of the same magnitude as those achieved in RCTs [6, 7]. Conversely, the treatment approach with RBZ still includes a large number of patients treated following a PRN approach, receiving a low number of injections but requiring high treatment burden (over 10 visits in 52 weeks). Mean gain in VA observed with RBZ in clinical practice was lower than the mean gains reported in the RCTs and not comparable in magnitude.
The SLR identified studies published between 2009 and 2018. Because the two drugs were introduced in clinical practice at different time points (RBZ was introduced in 2007 and intravitreally administered aflibercept in 2012) the publications describing the use and outcomes with intravitreally administered aflibercept are more recent while the publications describing the use and outcomes with RBZ range from 2009 to 2017. The authors compiled the VA gains reported by the individual RBZ studies to identify if there were significant differences/imbalances in the mean VA gain and in the clinical practice in RBZ studies completed both before and after 2015. The analysis of the individual data shows that the earlier studies (prior to 2015) report some of the highest and more consistent gains in visual acuity among the RBZ studies (Rothenbuehler et al. [36], Gillies et al. [41], Finger et al. [43], Gungel et al. [44], Inoue et al. [45]). (See Appendix 1D in the supplementary material.) The mean gain in VA estimated in this analysis is supported by the results from other RBZ real-world meta-analyses (Kim et al. [10], Guo et al. [11]) that estimated mean gains in VA at week 52 ranging between + 4.85 to + 5.00 letters. These estimates are in line with our findings (mean gain of + 4.48 letters).
Pivotal studies such as the VIEW trials were useful to establish the cost-effectiveness of intravitreally administered aflibercept and RBZ under RCT-like conditions, demonstrating that the same results can be achieved with lower resource use. This analysis provides new data and an insight into the real-world cost-effectiveness of these anti-VEGF agents. In current practice, intravitreally administered aflibercept is associated with higher mean gains in VA and lower treatment burden (overall injections and monitoring visits), which translate into a positive ICER despite higher acquisition costs. The scenarios tested here (different time horizons) and the sensitivity analyses (12-week effectiveness and probabilistic sensitivity analysis) confirm the robustness of the findings that, for a threshold of €30,000 per QALY and the French healthcare system, intravitreally administered aflibercept is a cost-effective treatment option for patients with nAMD. In a real-world setting, the cost-effectiveness analysis followed a similar approach to a previous published analysis in a US setting [53], showing that intravitreally administered aflibercept was cost-effective compared with RBZ, with better outcomes (i.e., higher gain in QALYs) despite slightly higher overall costs.
Limitations
Several potential limitations and strengths of the analyses should be considered. Our objective was to provide an overview of how the two drugs are used in clinical practice and how the clinical practice (e.g., treatment approach) relates to real-world effectiveness and ultimately to cost-effectiveness. The analysis provides an overview that is comprehensive and representative of the combined treatment approaches used in clinical practice to manage patients with nAMD. There is value in taking this comprehensive approach but there are also limitations. One important limitation being that it does not assess the impact of implementing specific treatment approaches (i.e., PRN and T&E). A key aspect to consider in future research is to assess the effectiveness and cost-effectiveness of aflibercept and RBZ when administered intravitreally following a T&E regimen. When correcting for treatment effect modifiers, the analysis included age and baseline visual acuity, but it did not correct for other known effect modifiers such as baseline choroidal neovascularization (CNV) lesion type and size. It was not possible to assess the impact of CNV because of insufficient data or low power.
The meta-analysis and multivariable meta-regression included estimates that were based on a limited number of observations. Although the studies included are representative of clinical practice and the patient population, including additional data points and more observations will improve the validity of the results and the extrapolation of conclusions. Uncertainty was also associated with the pooled estimates because of the missing SDs (i.e., large variability in imputed SDs leading to larger CIs of pooled outcomes). Furthermore, the meta-analysis and meta-regression relied on published articles and abstracts, which are not free from sample selection bias (e.g., reporting bias). In addition, the meta-analysis was based on aggregate (mean) data and not patient-level data, so it may be prone to ecological bias (i.e., the associations found between the variables of interest and outcomes at study level may not be the same at patient level). The multivariable meta-regression is potentially not free from confounding bias; because of the limited number of observations and limited availability of covariates, it was not possible to control for all potential covariates. Despite these limitations, there are several strengths to consider. Study selection in the SLR was based on strict criteria, resulting in a small number of observations; but it provided high-quality data, and the methodology followed recent recommendations, including undertaking an SLR and using multiple imputations and meta-regression. The use of multiple imputations ensured that the confidence error correctly reflected the uncertainties from the imputations. The meta-regression was useful to quantify the between-study heterogeneity explained by the explanatory variable.
Conclusions
The analysis identified clear differences in the overall treatment approach and how ophthalmologists use intravitreally administered aflibercept and RBZ in clinical practice. Ultimately, these differences influence the mean real-world effectiveness of the two drugs. Intravitreal treatment with aflibercept (injection frequency and patients follow-up) was consistent and in line with the European label recommendations. Patients treated intravitreally with aflibercept in clinical practice reported a mean gain in VA of similar magnitude to the mean gains in VA reported in the pivotal RCT. Conversely, treatment with RBZ varied significantly across the different studies. On average, RBZ-treated patients reported a low injection frequency and a frequent follow-up, driven in part by the high number of patients treated with PRN regimens. RBZ-treated patients reported gains in VA versus baseline; however, the magnitude of the gain in VA was not comparable to the VA gains reported in the RBZ pivotal RCT. Intravitreal treatment with aflibercept was associated with better mean VA outcomes and an incremental gain in QALYs compared with RBZ and can be considered cost-effective for the treatment of nAMD in patients in France despite a higher price for each individual intravitreal injection of aflibercept compared with RBZ.
References
Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–16.
Lucentis® [prescribing information]. https://www.gene.com/download/pdf/lucentis_prescribing.pdf. Accessed June 28, 2018.
Eylea® [prescribing information]. https://www.regeneron.com/sites/default/files/EYLEA_FPI.pdf. Accessed June 28, 2019.
Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–44.
Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–31.
Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–48.
Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121(1):193–201.
Holz FG, Tadayoni R, Beatty S, et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol. 2015;99(2):220–6.
Holz FG, Tadayoni R, Beatty S, et al. Key drivers of visual acuity gains in neovascular age-related macular degeneration in real life: findings from the AURA study. Br J Ophthalmol. 2016;100(12):1623–8.
Kim LN, Mehta H, Barthelmes D, Nguyen V, Gillies MC. Metaanalysis of real-world outcomes of intravitreal ranibizumab for the treatment of neovascular age-related macular degeneration. Retina. 2016;36(8):1418–31.
Guo MY, Etminan M, Cheng JZ, Zafari Z, Maberley DAL. One-year effectiveness study of intravitreous ranibizumab in wet (neovascular) age-related macular degeneration: a meta-analysis. Pharmacotherapy. 2018;38(2):197–204.
Weber M, Velasque L, Coscas F, Faure C, Aubry I, Cohen SY. Effectiveness and safety of intravitreal aflibercept in patients with wet age-related macular degeneration treated in routine clinical practices across France: 12-month outcomes of the RAINBOW study. BMJ Open Ophthalmol. 2019;4(1):e000109.
Talks JS, Lotery AJ, Ghanchi F, et al. First-year visual acuity outcomes of providing aflibercept according to the VIEW study protocol for age-related macular degeneration. Ophthalmology. 2016;123(2):337–43.
Framme C, Eter N, Hamacher T, et al. Aflibercept for patients with neovascular age-related macular degeneration in routine clinical practice in Germany. Ophthalmol Retina. 2018;2(6):539–49.
DeCroos FC, Reed D, Adam MK, et al. Treat-and-extend therapy using aflibercept for neovascular age-related macular degeneration: a prospective clinical trial. Am J Ophthalmol. 2017;180:142–50.
Almuhtaseb H, Johnston RL, Talks JS, Lotery AJ. Second-year visual acuity outcomes of nAMD patients treated with aflibercept: data analysis from the UK Aflibercept Users Group. Eye (Lond). 2017;31(11):1582–8.
Guo MY, Cheng J, Etminan M, Zafari Z, Maberley D. One year effectiveness study of intravitreal aflibercept in neovascular age-related macular degeneration: a meta-analysis. Acta Ophthalmol. 2018. https://doi.org/10.1111/aos.13825.
Elshout M, van der Reis MI, Webers CA, Schouten JS. The cost-utility of aflibercept for the treatment of age-related macular degeneration compared to bevacizumab and ranibizumab and the influence of model parameters. Graefes Arch Clin Exp Ophthalmol. 2014;252(12):1911–20.
Panchmatia HR, Clements KM, Hulbert E, et al. Aflibercept vs. ranibizumab: cost-effectiveness of treatment for wet age-related macular degeneration in Sweden. Acta Ophthalmol. 2016;94(5):441–8.
Ghosh W, Wickstead R, Claxton L, et al. The cost-effectiveness of ranibizumab treat and extend regimen versus aflibercept in the UK. Adv Ther. 2016;33(9):1660–76.
Claxton L, Hodgson R, Taylor M, Malcolm B, Pulikottil Jacob R. Simulation modelling in ophthalmology: application to cost effectiveness of ranibizumab and aflibercept for the treatment of wet age-related macular degeneration in the United Kingdom. Pharmacoeconomics. 2017;35(2):237–48.
Vottonen P, Kankaanpaa E. Cost-effectiveness of treating wet age-related macular degeneration at the Kuopio University Hospital in Finland based on a two-eye Markov transition model. Acta Ophthalmol. 2016;94(7):652–6.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12.
Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12.
van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):67.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(2):1–48.
Haute Authorité de Santé. 2012. Choices in methods for economic evaluation. https://www.has-sante.fr/portail/upload/docs/application/pdf/2012-10/choices_in_methods_for_economic_evaluation.pdf. Accessed June 28, 2019.
Quaranta M, Devin F, Quentel G, et al. Evaluation of time-to-recurrence of disease activity in treatment-naïve patients with neovascular age-related macular degeneration after ranibizumab treatment: 12-month analysis from the ORACLE study. Investig Ophthalmol Vis Sci. 2017;58(8):900.
van der Pols JC, Bates CJ, McGraw PV, et al. Visual acuity measurements in a national sample of British elderly people. Br J Ophthalmol. 2000;84(2):165–70.
Duval MV, Rougier MB, Delyfer MN, Combillet F, Korobelnik JF. Reponse visuelle et anatomique en condition de «raie vie» du traitement par aflibercept chez les patients naifs atteints de degenerescence maculaire liee a l’age exsudative (Real life visual and anatomic outcomes of aflibercept treatment for treatment-naive patients with exudative age-related macular degeneration). J Fr Ophtalmol. 2017;40(4):270–8.
Boulanger-Scemama E, Sayag D, Ha Chau Tran T, et al. Ranibizumab et degenerescence maculaire liee a l’age exsudative: analyse multicentrique a 5ans des resultats fonctionnels et anatomiques en pratique clinique reelle (Ranibizumab and exudative age-related macular degeneration: 5-year multicentric functional and anatomical results in real-life practice). J Fr Ophtalmol. 2016;39(8):668–74.
Czoski-Murray C, Carlton J, Brazier J, Young T, Papo NL, Kang HK. Valuing condition-specific health states using simulation contact lenses. Value Health. 2009;12(5):793–9.
Lee AY, Lee CS, Egan CA, et al. UK AMD/DR EMR REPORT IX: comparative effectiveness of predominantly as needed (PRN) ranibizumab versus continuous aflibercept in UK clinical practice. Br J Ophthalmol. 2017;101(12):1683–8.
Gillies MC, Nguyen V, Daien V, Arnold JJ, Morlet N, Barthelmes D. Twelve-month outcomes of ranibizumab vs aflibercept for neovascular age-related macular degeneration: data from an observational study. Ophthalmology. 2016;123(12):2545–53.
Rothenbuehler SP, Waeber D, Brinkmann CK, Wolf S, Wolf-Schnurrbusch UE. Effects of ranibizumab in patients with subfoveal choroidal neovascularization attributable to age-related macular degeneration. Am J Ophthalmol. 2009;147(5):831–7.
Kumar A, Sahni JN, Stangos AN, Campa C, Harding SP. Effectiveness of ranibizumab for neovascular age-related macular degeneration using clinician-determined retreatment strategy. Br J Ophthalmol. 2011;95(4):530–3.
Mariani A, Deli A, Ambresin A, Mantel I. Characteristics of eyes with secondary loss of visual acuity receiving variable dosing ranibizumab for neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2011;249(11):1635–42.
Williams TA, Blyth CP. Outcome of ranibizumab treatment in neovascular age related macula degeneration in eyes with baseline visual acuity better than 6/12. Eye (Lond). 2011;25(12):1617–21.
Wickremasinghe SS, Xie J, Guymer RH, Wong TY, Kawasaki R, Qureshi S. Retinal vascular changes following intravitreal ranibizumab injections for neovascular AMD over a 1-year period. Eye (Lond). 2012;26(7):958–66.
Gillies MC, Walton R, Simpson JM, et al. Prospective audit of exudative age-related macular degeneration: 12-month outcomes in treatment-naive eyes. Invest Ophthalmol Vis Sci. 2013;54(8):5754–60.
Ross AH, Donachie PH, Sallam A, et al. Which visual acuity measurements define high-quality care for patients with neovascular age-related macular degeneration treated with ranibizumab? Eye (Lond). 2013;27(1):56–64.
Finger RP, Guymer RH, Gillies MC, Keeffe JE. The impact of anti-vascular endothelial growth factor treatment on quality of life in neovascular age-related macular degeneration. Ophthalmology. 2014;121(6):1246–51.
Gungel H, Osmanbasoglu OA, Altan C, Baylancicek DO, Pasaoglu IB. The effects of ranibizumab injections on fluorescein angiographic findings and visual acuity recovery in age-related macular degeneration. Clin Ophthalmol. 2014;8:981–8.
Inoue M, Arakawa A, Yamane S, Kadonosono K. Intravitreal injection of ranibizumab using a pro re nata regimen for age-related macular degeneration and vision-related quality of life. Clin Ophthalmol. 2014;8:1711–6.
Pagliarini S, Beatty S, Lipkova B, et al. A 2-year, phase IV, multicentre, observational study of ranibizumab 0.5 mg in patients with neovascular age-related macular degeneration in routine clinical practice: the EPICOHORT study. J Ophthalmol. 2014;2014:857148.
Basheer K, Mensah E, Khanam T, Minakaran N. Visual outcomes of age-related macular degeneration patients undergoing intravitreal ranibizumab monotherapy in an urban population. Clin Ophthalmol. 2015;9:959–65.
Piermarocchi S, Miotto S, Colavito D, Leon A, Segato T. Combined effects of genetic and non-genetic risk factors affect response to ranibizumab in exudative age-related macular degeneration. Acta Ophthalmol. 2015;93(6):e451–7.
Rana-Rahman RL, Kotagiri A, Habib M, et al. Real life clinical audit on neo-vascular age related macular degeneration (nAMD)—comparing fixed dose aflibercept versus PRN ranibizumab (RBZ) over 12 months. Investig Ophthalmol Vis Sci. 2015;56(7):4618.
Guymer RH, Macfadden W. Baseline characteristics and 1-year outcomes of patients with neovascular age-related macular degeneration from the real-world LUMINOUS™ study: global results vs Australian cohort. Investig Ophthalmol Vis Sci. 2016;57(12):531.
Wickremasinghe SS, Janakan V, Sandhu SS, Amirul-Islam FM, Abedi F, Guymer RH. Implication of recurrent or retained fluid on optical coherence tomography for visual acuity during active treatment of neovascular age-related macular degeneration with a treat and extend protocol. Retina. 2016;36(7):1331–9.
Barthelmes D, Nguyen V, Daien V, et al. Two year outcomes of “treat and extend” intravitreal therapy using aflibercept preferentially for neovascular age-related macular degeneration. Retina. 2018;38(1):20–8.
Hernandez L, Lanitis T, Cele C, Toro-Diaz H, Gibson A, Kuznik A. Intravitreal aflibercept versus ranibizumab for wet age-related macular degeneration: a cost-effectiveness analysis. J Manag Care Spec Pharm. 2018;24(7):608–16.
Acknowledgements
The authors wish to acknowledge Daniel Janer, Walter Bouwmeester, Ling-Hsiang Chuang for their contribution to this project.
Funding
This study was funded by Bayer AG, Basel. Bayer AG, Basel also funded the Journals Rapid Service and Open Access fees.
Medical Writing and/or Editorial Assistance
Medical writing assistance was provided by Corey Eagan, MPH, of PAREXEL, and Juliet Fawcett, PhD, of Juliet Fawcett Medical Writing Ltd, and was funded by Bayer AG, Basel.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Disclosures
Joao Carrasco is a paid employee of Bayer Consumer Care AG, Basel, Switzerland. Georg-Alexander Pietsch is a paid employee of Bayer Consumer Care AG, Basel, Switzerland. Marie-Pierre Nicolas was a paid employee of Bayer HealthCare SAS, France, at the time of this research. Cecile Koerber was a paid employee of Bayer HealthCare SAS, France, at the time of this research. At the time of this research, Jisu Yoon was a paid employee of Pharmerit International, which was contracted by Bayer to undertake the data analysis for this study. Craig Bennison was a paid employee of Pharmerit International, which was contracted by Bayer to undertake the data analysis for this study.
Compliance with Ethics Guidelines
This study is based on previously conducted studies and does not involve any new studies of human participants or animal subjects performed by any of the authors. This study did not require ethical approval as it did not involve human participants or animal subjects.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.10098272.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Carrasco, J., Pietsch, GA., Nicolas, MP. et al. Real-World Effectiveness and Real-World Cost-Effectiveness of Intravitreal Aflibercept and Intravitreal Ranibizumab in Neovascular Age-Related Macular Degeneration: Systematic Review and Meta-Analysis of Real-World Studies. Adv Ther 37, 300–315 (2020). https://doi.org/10.1007/s12325-019-01147-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-019-01147-6