Abstract
Fungi are responsible for around 20% of microbiologically documented infections in intensive care units (ICU). In the last decade, the incidence of invasive fungal infections (IFI), including candidemia, has increased steadily because of increased numbers of both immunocompromised and ICU patients. To improve the outcomes of patients with IFI, intensivists need to be aware of the inherent challenges. This narrative review summarizes the features of routinely used treatments directed against IFI in non-neutropenic ICU patients, which include three classes of antifungals: polyenes, azoles, and echinocandins. ICU patients’ pathophysiological changes are responsible for deep changes in the pharmacokinetics of antifungals. Moreover, drug interactions affect the response to antifungal treatments. Consequently, appropriate antifungal dosage is a challenge under these special conditions. Dosages should be based on renal and liver function, and serum concentrations should be monitored. This review summarizes recent guidelines, focusing on bedside management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fungi are responsible for approximately 20% of microbiologically documented intensive care unit (ICU) infections [1]. In the last decade, the incidence of invasive fungal infections (IFI), including candidemia, has steadily increased as a result of the increasing numbers of both immunocompromised and critically ill patients [2,3,4,5,6].
Candida spp. are the third leading cause of infections in the ICU, accounting for 90% of fungal infections [1]. Invasive candidiasis (IC) includes bloodstream and deep infections caused by the Candida species. Studies have shown a cumulative incidence of 7.07 episodes of IC per 1000 ICU admissions [7]. Of note, over recent decades the incidence of Candida albicans infections has decreased with a relative increase in non-albicans Candida infections, including the fast emergence of Candida auris [8,9,10]. In ICU patients, IC became a challenge with a mortality rate approaching 40% [11]. Although attributable mortality is difficult to establish, candidemia has been identified as an independent predictor of mortality after controlling for confounders [12]. Delay in initiating adequate antifungal treatment is associated with increased mortality. Remarkably, antifungal treatment recommendations remain largely based on randomized clinical trials that were not restricted to ICU patients.
Invasive aspergillosis (IA) is an opportunistic infection that occurs mainly in patients with prolonged periods of neutropenia with or without hematological malignancies, patients who underwent allogeneic stem cell transplantation or solid organ transplantation, and patients with HIV/AIDS. In recent years, however, IA has increasingly been recognized as an emerging disease in non-neutropenic individuals, including patients with chronic obstructive pulmonary disease and other chronic lung or connective tissue diseases requiring corticosteroid therapy, decompensated liver cirrhosis or acute liver failure, solid cancer, chronic renal failure with replacement therapy, diabetes, severe influenza, and even ICU patients without any risk factors apart from a prolonged stay [13].
The incidence of Aspergillus spp. infections ranges from 0.3% to 6.9% in ICU patients [14]. Prompt administration of an effective treatment for IA is critical to reduce the mortality rate, which ranges from 60% to 90% [14]. Intensivists need to be aware of the specific risk factors for IFI as well as the diagnostic and therapeutic challenges to improve outcome.
The aim of this narrative review was to summarize clinically relevant knowledge on the currently used antifungals, focusing on non-neutropenic ICU patients. These patients are indeed at risk of IFI because of pathophysiological changes that influence drug pharmacokinetics (PK).
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Antifungal Drug Classes
Since the 1980s there has been an increasing but limited discovery of antifungal agents [15, 16]. The three principal classes of antifungal agents are polyenes, azoles, and echinocandins. Details on pharmacokinetics are provided in Table 1. Table 2 summarizes the features of antifungals in patients with renal or liver failure.
Polyenes
Polyenes (amphotericin B and nystatin) act in the fungal membrane by binding to ergosterol and causing disruption of the membrane structure promoting extravasation of intracellular constituents and, consequently, cell death (Fig. 1) [17]. They have a broad spectrum of action, fungicidal activity, and an activity against most Candida, most Aspergillus, and Mucorales species. However, many Candida lusitaniae and Aspergillus terreus strains are resistant to amphotericin B (Tables 3, 4).
Mechanism of action of traditional antifungal agents on cellular targets. Azoles inhibit the ergosterol synthesis in the endoplasmic reticulum of the fungal cell. They act by interfering with the enzyme lanosterol 14-alpha demethylase, involved in the transformation of lanosterol into ergosterol. Polyenes act in the fungal membrane by binding to ergosterol and causing disruption of the membrane structure, promoting extravasation of intracellular constituents and consequent cell death. Echinocandins inhibit 1,3-beta-d-glucan synthase, thereby preventing synthesis of glucan, which is present in the cell membrane of fungi [18]
The standard amphotericin B formulation is associated with renal toxicity, caused by the vasoconstriction of the afferent arteriole, resulting in reduced renal blood flow and glomerular filtration rate combined with tubular injury, causing loss of potassium, magnesium bicarbonates, and amino acids. To reduce renal injury, liposomal amphotericin B allows lower absorption of amphotericin B by the reticuloendothelial system, resulting in a longer stay in the bloodstream.
Amphotericin B is contraindicated in patients with renal failure. Liposomal amphotericin B and amphotericin B lipid complex are less nephrotoxic than conventional amphotericin B, allowing a higher dosage because their PK are very different [20]. Since enteral absorption is negligible for all commercially available amphotericin B formulations, they must be administered by intravenous infusion.
Infusion-related adverse events include chills, rigor, fever, hypotension or hypertension, hypoxia, nausea, vomiting, and hypokalemia, and affect about half of patients treated with conventional amphotericin B. The adverse event mechanisms are driven by activation of proinflammatory pathways [21,22,23].
Azoles
Azoles act by inhibiting ergosterol synthesis in the endoplasmic reticulum of the fungal cell (Fig. 1). They have fungistatic properties affecting cell growth and proliferation. Candida krusei and Candida glabrata strains may show resistance against azoles (Table 3) [24]; however, a large accumulation of toxic sterols can eventually lead to fungal cell death [25, 26].
Triazoles include fluconazole, itraconazole, voriconazole, posaconazole, and isavuconazole. The most frequent side effects induced by triazoles include liver toxicity, prolonged QTc, and emerging resistance among fungal isolates [27]. Moreover, triazoles inhibit most of the cytochrome P450 enzymes (including the CYP34A), inducing variable drug–drug interactions. This plays a key role in metabolizing immunosuppressant drugs such as cyclosporine, tacrolimus, and sirolimus [28]. Thus, co-administration of a triazole with these immunosuppressant drugs increases the risk of toxicity, or upon discontinuation, increases the risk of rejection or graft-versus-host disease. Close therapeutic drug monitoring of both immunosuppressants and triazoles is therefore indispensable.
Fluconazole
Fluconazole is available for intravenous and oral administration with high bioavailability. It is active on most Candida species and is usually well tolerated. ICU patients treated with fluconazole should receive a loading dose (12 mg/kg) followed by a maintenance dose (6 mg/kg) [29]. This dosage is supported because of impaired target site penetration in septic patients [30]. For obese ICU patients, fluconazole dosage should be based on actual body weight [31]. For patients with renal failure (creatinine clearance 11–50 mL/min) it is necessary to reduce the maintenance dose by 50% because of delayed elimination [32]. Large amounts of fluconazole are eliminated by renal replacement therapy.
Voriconazole
Voriconazole has high bioavailability and is available for intravenous and oral administration. It has a broad antifungal spectrum and is active against most Candida and Aspergillus species. Voriconazole is recommended as first-line treatment for IA because it had better clinical outcomes than amphotericin B deoxycholate in an open-label randomized clinical trial [33].
Renal failure has no relevant influence on voriconazole PK, but a considerable accumulation of the solvent sulfobutylether-β-cyclodextrin (SBECD) was found in patients with renal failure requiring intravenous administration of voriconazole. This solvent is a large cyclic oligosaccharide that is potentially nephrotoxic at high concentrations. The manufacturer recommends oral administration in patients with a creatinine clearance below 50 mL/min. Of note, in solid organ transplant patients, the significant interaction of voriconazole with sirolimus contraindicates there concomitant use [32].
Posaconazole
Posaconazole has a wide antimycotic spectrum, including activity against Mucorales, and is licensed for antifungal prophylaxis in selected hematological high-risk patient. For a decade, posaconazole was available only as an oral suspension that displayed poor and highly variable absorption. An intravenous formulation and a tablet with improved bioavailability are now available. Posaconazole is a strong inhibitor of CYP3A4, which is responsible for drug–drug interactions. In a study that included ICU patients, the majority had subtherapeutic serum concentrations during treatment with standard doses of oral suspension [34]. Mild to moderate renal or liver impairment had no relevant influence on posaconazole’s PK.
Isavuconazole
Isavuconazole is a new triazole agent that can be given once a day and offers a wider spectrum of antifungal activity than voriconazole, including activity against most Mucorales. It has an excellent bioavailability and predictable PK. It can be used in patients with renal failure given the absence of cyclodextrin in the intravenous formulation. A large double-blind randomized clinical trial showed non-inferiority for isavuconazole versus voriconazole in terms of all-cause mortality when used as a primary treatment for invasive fungal disease caused by Aspergillus species or other filamentous fungi [35]. In addition, a matched case–control analysis of isavuconazole versus amphotericin B provided evidence for the efficacy and superior safety profile of isavuconazole in the treatment of mucormycosis [36]. The most commonly reported side effects include gastrointestinal disorders such as nausea, vomiting, and diarrhea. A recent double-blind randomized clinical trial did not show non-inferiority of isavuconazole to caspofungin for primary treatment of IC. Secondary endpoints were similar between both groups [37].
Gastrointestinal disorders and central nervous adverse effects are possible during isavuconazole treatment. Whereas prolongation of the QT interval is a common adverse effect of azole antifungals, shortening of the QT interval has been observed with isavuconazole [38]. Because isavuconazole is a moderate CYP3A4 inhibitor, interactions with immunosuppressants are reported to be less pronounced than those with voriconazole. However, increased serum concentrations of cyclosporine A, tacrolimus, sirolimus, and mycophenolate mofetil must be anticipated when isavuconazole is co-administered.
Echinocandins
The echinocandins belong to a class of semisynthetic lipopeptides that inhibit the synthesis of the 1,3-beta-d-glucan component of the fungi cell wall (Fig. 1). Echinocandins have a broad spectrum of fungicidal activity against the Candida species, and fungistatic activity against most Aspergillus species [39] (Tables 3, 4). Limitations for use of currently approved echinocandins include the absence of an oral formulation. Frequently reported side effects include headache, nausea, diarrhea, phlebitis, and pruritus. Severe side effects such as leukopenia, neutropenia, anemia, hypokalemia, and liver toxicity are rarely reported [40,41,42].
Caspofungin
The standard dose is 70 mg as a single loading dose followed by a maintenance dose of 50 mg once a day or 70 mg once a day when body weight exceeds 80 kg. It displays linear PK. Immediately after infusion caspofungin undergoes rapid distribution into tissue, mainly the liver. About 95% of caspofungin is typically bound to plasma proteins and it is metabolized in the liver. For non-ICU patients with moderate hepatic impairment (Child–Pugh score 7–9), reducing the maintenance dose to 35 mg per day is recommended [43]. ICU patients with moderate liver failure may achieve subtherapeutic caspofungin exposure with the adjusted dose of 35 mg per day. The authors ascribed the low concentrations to typical physiological alterations occurring in ICU patients (hypoalbuminemia) [44] and recommended standard doses. Since caspofungin elimination is largely independent from renal function, standard doses are suggested in patients with renal failure, even those with terminal renal failure requiring hemodialysis [45,46,47,48,49].
Anidulafungin
Anidulafungin is licensed for the treatment of IC in adult patients. The recommended dose is 200 mg once a day on day 1 (loading dose) and then 100 mg daily (maintenance dose). Renal failure has no influence on anidulafungin elimination [40]. Unlike caspofungin, liver failure results in decreased exposure for anidulafungin; no dose adjustment is recommended. An increased degradation due to reduced protein binding and an enlarged volume of distribution has been suggested [50].
Micafungin
Micafungin was shown to be as effective as both l-amphotericin B and caspofungin in randomized clinical trials [51, 52]. However, the potential risk for developing liver tumors indicates that use should be restricted to when other antifungals are not appropriate.
Pharmacokinetics Features
ICU Patients
ICU patients, particularly those on broad-spectrum antimicrobial treatment, renal replacement therapy, total parenteral nutrition, or corticosteroid or other immunosuppressive agents, are at risk of IC. Timely and sufficient exposure to appropriate antifungals is required to eradicate fungus. Inadequate initial antifungal doses contribute to both poor outcomes [53,54,55] and emergence of resistance [26].
ICU patients have pathophysiological changes that are responsible for antifungal PK alterations: organ failure, reduced protein binding, capillary leakage resulting in an altered drug volume of distribution, and use of organ support (i.e., renal replacement therapy and/or extracorporeal membrane oxygenation, ECMO). Moreover, interacting co-medications may result in variable PK of antifungals [56]. In ICU patients, PK may therefore be very different from PK of less compromised patients. Appropriate dosage of antifungals is challenging under these special conditions, because respective PK data are sparse.
The doses determined by data extracted from other patient groups are suboptimal. Investigators have attempted to assess the PK variability of antifungals. A multinational patient study defining antibiotic levels in the intensive care unit (DALI) found considerable intervariability with fluconazole, anidulafungin, and caspofungin, indicating that a large number of patients do not receive adequate treatment [57]. Those results confirmed previous findings, suggesting the need for routine monitoring of antifungal serum concentrations [50, 58].
Recommendations of Recent Guidelines
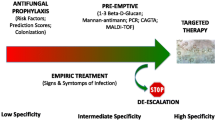
IC encompasses three entities: candidemia in the absence of deep-seated candidiasis, candidemia associated with deep-seated candidiasis, and deep-seated candidiasis in the absence of candidemia [59]. The most recent guidelines for IC management were provided by the Infectious Diseases Society of America (IDSA) in 2016 and by a task force of the European Society of Intensive Care Medicine–European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in 2019 [29, 60]. The latter specifically focused on ICU patients (Fig. 2). Both documents came out against the universal use of antifungals in patients without clear signs or symptoms of infections (prophylaxis). However, guidelines from IDSA posed a weak recommendation (moderate quality evidence) for use of fluconazole prophylaxis in high-risk patients in ICUs with IC rates above 5% [29, 60, 61]. Both documents supported the use of empirical antifungal treatment (based on signs or symptoms of infections without certain proof of candida infections) only in carefully selected patients with a high risk of IC, and in conjunction with other diagnostic tools and data (e.g., biomarkers such as beta-d-glucan; culture data from nonsterile sites) [29, 60]. Moreover, only patients with septic shock, multiple organ failure, no other causes of fever, and more than one extradigestive site of Candida spp. colonization (e.g., urine, mouth, throat, upper and lower respiratory tracts, skin folds, drains, operative site) should receive empirical antifungal treatment with an echinocandin as the first-line agent (strong recommendation; low-quality evidence) [29]. Fluconazole can be used in less severe patients (no septic shock and/or multiple organ failure) and in settings with a low rate of fluconazole resistance. Echinocandins should also be used in patients who are likely to be infected or colonized by fluconazole-resistant Candida spp. (i.e., Candida krusei, Candida glabrata). This regimen was also recommended for the targeted treatment of IC [29, 60].
Practical guidelines for empiric and curative treatment of candidiasis adapted from the IDSA guideline [60]
Transition from echinocandin to fluconazole (de-escalation) is recommended in clinically stable patients who have an isolate susceptible to fluconazole [29] and have negative repeated blood cultures following the initiation of antifungal treatment [60]. The de-escalation should not be considered in cases of difficult or impossible source control (e.g., removing central venous catheter, surgery for intra-abdominal candidiasis). The recommended treatment duration of candidemia without metastatic complications is 14 days after the first negative blood culture [29, 60], taken daily after the initiation of targeted treatment [60]. In case of inadequate source control (e.g., no removal of central venous catheter, no definitive surgical control or drainage for intra-abdominal candidiasis, endocarditis) the duration of therapy should be individualized [29]. The lipid formulation amphotericin B (3–5 mg per kg per day) is recommended for infections by azole- and echinocandin-resistant strains [60]. Of note, a recent meta-analysis found no evidence of a therapeutic or survival benefit from choosing between echinocandins, voriconazole, or amphotericin B formulations as first-line therapy for ICU adults with invasive infection of the Candida species [62].
Regarding IA infections in ICU patients, the 2018 ESCMID–European Confederation of Medical Microbiology–European Respiratory Society guidelines underlined the difficulty of diagnosis in ICU patients and suggested that early diagnosis and treatment of IA should be based on the integration of clinical findings, risk factors, radiological data, and microbiological data [63]. A high cutoff (optical density index 0.5–1) of galactomannan, a pan-fungal antigen, in the bronchoalveolar lavage can be considered in decisions regarding when to start therapy [63]. Thin-section chest computerized tomography is the imaging of choice, but classic signs are rare in ICU patients, who usually present non-specific radiological findings [63]. It is still unclear if a prophylactic treatment may be cost-effective in high-risk non-neutropenic ICU patients. However, common risk factors to consider for Aspergillosis in the ICU are chronic obstructive pulmonary disease, steroid treatment, sepsis, and influenza-associated respiratory failure [63]. The first-line agent is voriconazole (2 × 6 mg per kg on day 1 and then 2 × 4 mg per kg intravenously) (Table 5). Combination with an echinocandin can be considered but the quality of the supporting evidence is low. Susceptibility testing is recommended for voriconazole and therapeutic drug monitoring in case of treatment failure [63].
Conclusion
Fungi infections are an increasing concern in ICU patients and have led to the development of recent guidelines. Three treatment families are currently used for fungi infections, but because of the specific pathophysiological status of ICU patients, PK data on those treatments are still scarce and numerous questions remain. Therapeutic drug monitoring is an essential option for evaluating treatment efficacy and tolerance.
References
Vincent J-L, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–9.
Sague CMB, Jarvis WR, National Nosocomial Infections Surveillance System. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980–1990. J Infect Dis. 1993;167:1247–51.
Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–17.
Bassetti M, Righi E, Costa A, et al. Epidemiological trends in nosocomial candidemia in intensive care. BMC Infect Dis. 2006;6. http://bmcinfectdis.biomedcentral.com/articles/10.1186/1471-2334-6-21. Accessed 6 Sept 2019.
Leroy O, Gangneux J-P, Montravers P, et al. Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: a multicenter, prospective, observational study in France (2005–2006). Crit Care Med. 2009;37:1612–8.
Kett DH, Azoulay E, Echeverria PM, Vincent J-L, Extended Prevalence of Infection in ICU Study (EPIC II) Group of Investigators. Candida bloodstream infections in intensive care units: analysis of the extended prevalence of infection in intensive care unit study. Crit Care Med. 2011;39:665–70.
Bassetti M, Giacobbe DR, Vena A, et al. Incidence and outcome of invasive candidiasis in intensive care units (ICUs) in Europe: results of the EUCANDICU project. Crit Care. 2019;23:219.
Pfaller MA, Moet GJ, Messer SA, Jones RN, Castanheira M. Geographic variations in species distribution and echinocandin and azole antifungal resistance rates among Candida bloodstream infection isolates: report from the SENTRY Antimicrobial Surveillance Program (2008 to 2009). J Clin Microbiol. 2011;49:396–9.
Holley A, Dulhunty J, Blot S, et al. Temporal trends, risk factors and outcomes in albicans and non-albicans candidaemia: an international epidemiological study in four multidisciplinary intensive care units. Int J Antimicrob Agents. 2009;33(554):e1–7.
Cortegiani A, Misseri G, Fasciana T, Giammanco A, Giarratano A, Chowdhary A. Epidemiology, clinical characteristics, resistance, and treatment of infections by Candida auris. J Intensive Care. 2018;6:69.
Delaloye J, Calandra T. Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence. 2014;5:161–9.
Lundberg JS, Perl TM, Wiblin T, et al. Septic shock: an analysis of outcomes for patients with onset on hospital wards versus intensive care units. Crit Care Med. 1998;26:1020–4.
Bassetti M, Bouza E. Invasive mould infections in the ICU setting: complexities and solutions. J Antimicrob Chemother. 2017;72:i39–47.
Dimopoulos G, Frantzeskaki F, Poulakou G, Armaganidis A. Invasive aspergillosis in the intensive care unit. Ann N Y Acad Sci. 2012;1272:31–9.
Sardi JCO, Scorzoni L, Bernardi T, Fusco-Almeida AM, Mendes Giannini MJS. Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J Med Microbiol. 2013;62:10–24.
Paramythiotou E, Frantzeskaki F, Flevari A, Armaganidis A, Dimopoulos G. Invasive fungal infections in the ICU: how to approach, how to treat. Molecules. 2014;19:1085–119.
Mesa-Arango AC, Scorzoni L, Zaragoza O. It only takes one to do many jobs: amphotericin B as antifungal and immunomodulatory drug. Front Microbiol. 2012;3:286.
de Oliveira Santos GC, Vasconcelos CC, Lopes AJO, et al. Candida infections and therapeutic strategies: mechanisms of action for traditional and alternative agents. Front Microbiol. 2018;9:1351.
Arendrup MC. Update on antifungal resistance in Aspergillus and Candida. Clin Microbiol Infect. 2014;20(Suppl 6):42–8.
Bellmann R. Clinical pharmacokinetics of systemically administered antimycotics. Curr Clin Pharmacol. 2007;2:37–58.
Walsh TJ, Finberg RW, Arndt C, et al. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. National Institute of Allergy and Infectious Diseases Mycoses Study Group. N Engl J Med. 1999;340:764–71.
Laniado-Laborín R, Cabrales-Vargas MN. Amphotericin B: side effects and toxicity. Rev Iberoam Micol. 2009;26:223–7.
Roden MM, Nelson LD, Knudsen TA, et al. Triad of acute infusion-related reactions associated with liposomal amphotericin B: analysis of clinical and epidemiological characteristics. Clin Infect Dis. 2003;36:1213–20.
Berkow EL, Lockhart SR. Fluconazole resistance in Candida species: a current perspective. Infect Drug Resist. 2017;10:237–45.
Shapiro RS, Robbins N, Cowen LE. Regulatory circuitry governing fungal development, drug resistance, and disease. Microbiol Mol Biol Rev. 2011;75:213–67.
Prasad R, Shah AH, Rawal MK. Antifungals: mechanism of action and drug resistance. Adv Exp Med Biol. 2016;892:327–49.
Carrillo-Muñoz AJ, Giusiano G, Ezkurra PA, Quindós G. Antifungal agents: mode of action in yeast cells. Rev Espanola Quimioter. 2006;19:130–9.
Groll AH, Townsend R, Desai A, et al. Drug–drug interactions between triazole antifungal agents used to treat invasive aspergillosis and immunosuppressants metabolized by cytochrome P450 3A4. Transpl Infect Dis. 2017;19:e12751.
Martin-Loeches I, Antonelli M, Cuenca-Estrella M, et al. ESICM/ESCMID task force on practical management of invasive candidiasis in critically ill patients. Intensive Care Med. 2019;45:789–805.
Sinnollareddy MG, Roberts MS, Lipman J, et al. In vivo microdialysis to determine subcutaneous interstitial fluid penetration and pharmacokinetics of fluconazole in intensive care unit patients with sepsis. Antimicrob Agents Chemother. 2016;60:827–32.
Momper JD, Capparelli EV, Wade KC, et al. Population pharmacokinetics of fluconazole in premature infants with birth weights less than 750 grams. Antimicrob Agents Chemother. 2016;60:5539–45.
Bellmann R, Smuszkiewicz P. Pharmacokinetics of antifungal drugs: practical implications for optimized treatment of patients. Infection. 2017;45:737–79.
Herbrecht R, Denning DW, Patterson TF, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347:408–15.
Ray J, Campbell L, Rudham S, Nguyen Q, Marriott D. Posaconazole plasma concentrations in critically ill patients. Ther Drug Monit. 2011;33:387–92.
Slavin MA, Thursky KA. Isavuconazole: a role for the newest broad-spectrum triazole. Lancet. 2016;387:726–8.
Marty FM, Ostrosky-Zeichner L, Cornely OA, et al. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect Dis. 2016;16:828–37.
Kullberg BJ, Viscoli C, Pappas PG, et al. Isavuconazole versus caspofungin in the treatment of candidemia and other invasive candida infections: the ACTIVE trial. PubMed—NCBI. https://www.ncbi.nlm.nih.gov/pubmed/30289478. Accessed 12 Sept 2019.
Mellinghoff SC, Bassetti M, Dörfel D, et al. Isavuconazole shortens the QTc interval. Mycoses. 2018;61:256–60.
Nett JE, Andes DR. Antifungal agents: spectrum of activity, pharmacology, and clinical indications. Infect Dis Clin North Am. 2016;30:51–83.
European Medicines Agency. Ecalta. EMA. 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/ecalta. Accessed 6 Sept 2019.
European Medicines Agency. Mycamine. EMA. 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/mycamine. Accessed 6 Sept 2019.
European Medicines Agency. Cancidas (previously Caspofungin MSD). EMA. 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/cancidas-previously-caspofungin-msd. Accessed 6 Sept 2019.
Mistry GC, Migoya E, Deutsch PJ, et al. Single- and multiple-dose administration of caspofungin in patients with hepatic insufficiency: implications for safety and dosing recommendations. J Clin Pharmacol. 2007;47:951–61.
Martial LC, Brüggemann RJM, Schouten JA, et al. Dose reduction of caspofungin in intensive care unit patients with Child Pugh B will result in suboptimal exposure. Clin Pharmacokinet. 2016;55:723–33.
Spriet I, Meersseman W, Annaert P, de Hoon J, Willems L. Pharmacokinetics of caspofungin in a critically ill patient with liver cirrhosis. Eur J Clin Pharmacol. 2011;67:753–5.
Nguyen TH, Hoppe-Tichy T, Geiss HK, et al. Factors influencing caspofungin plasma concentrations in patients of a surgical intensive care unit. J Antimicrob Chemother. 2007;60:100–6.
van der Elst KCM, Veringa A, Zijlstra JG, et al. Low caspofungin exposure in patients in intensive care units. Antimicrob Agents Chemother. 2017;61:e01582.
Muilwijk EW, Schouten JA, van Leeuwen HJ, et al. Pharmacokinetics of caspofungin in ICU patients. J Antimicrob Chemother. 2014;69:3294–9.
Stone EA, Fung HB, Kirschenbaum HL. Caspofungin: an echinocandin antifungal agent. Clin Ther. 2002;24:351–77 (discussion 329).
Dowell JA, Stogniew M, Krause D, Damle B. Anidulafungin does not require dosage adjustment in subjects with varying degrees of hepatic or renal impairment. J Clin Pharmacol. 2007;47:461–70.
Kuse E-R, Chetchotisakd P, da Cunha CA, et al. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet. 2007;369:1519–27.
Pappas PG, Rotstein CMF, Betts RF, et al. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin Infect Dis. 2007;45:883–93.
Zilberberg MD, Kollef MH, Arnold H, et al. Inappropriate empiric antifungal therapy for candidemia in the ICU and hospital resource utilization: a retrospective cohort study. BMC Infect Dis. 2010;10:150.
Andes D, Ambrose PG, Hammel JP, et al. Use of pharmacokinetic-pharmacodynamic analyses to optimize therapy with the systemic antifungal micafungin for invasive candidiasis or candidemia. Antimicrob Agents Chemother. 2011;55:2113–21.
Labelle AJ, Micek ST, Roubinian N, Kollef MH. Treatment-related risk factors for hospital mortality in Candida bloodstream infections. Crit Care Med. 2008;36:2967–72.
Roberts JA, Abdul-Aziz MH, Lipman J, et al. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis. 2014;14:498–509.
Sinnollareddy MG, Roberts JA, Lipman J, et al. Pharmacokinetic variability and exposures of fluconazole, anidulafungin, and caspofungin in intensive care unit patients: data from multinational Defining Antibiotic Levels in Intensive care unit (DALI) patients study. Crit Care. 2015;19:33.
Dowell JA, Knebel W, Ludden T, Stogniew M, Krause D, Henkel T. Population pharmacokinetic analysis of anidulafungin, an echinocandin antifungal. J Clin Pharmacol. 2004;44:590–8.
Clancy CJ, Nguyen MH. Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis. 2013;56:1284–92.
Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1–50.
Cortegiani A, Russotto V, Giarratano A. Associations of antifungal treatments with prevention of fungal infection in critically ill patients without neutropenia. JAMA. 2017;317:311–2.
Keane S, Geoghegan P, Povoa P, Nseir S, Rodriguez A, Martin-Loeches I. Systematic review on the first line treatment of amphotericin B in critically ill adults with candidemia or invasive candidiasis. Expert Rev Anti Infect Ther. 2018;16:839–47.
Ullmann AJ, Aguado JM, Arikan-Akdagli S, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect. 2018;24(Suppl 1):e1–38.
Acknowledgements
Funding
No funding or sponsorship was received for the publication of this study.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Jeanne Chatelon, Andrea Cortegiani, Emmanuelle Hammad, Nadim Cassir, and Marc Leone have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.9920075.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Chatelon, J., Cortegiani, A., Hammad, E. et al. Choosing the Right Antifungal Agent in ICU Patients. Adv Ther 36, 3308–3320 (2019). https://doi.org/10.1007/s12325-019-01115-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-019-01115-0