Abstract
Introduction
This exploratory study characterized the performance of a nasal dilator strip with improved spring forces in lowering nasal resistance during sleep and reducing sleep-disordered breathing in subjects with difficulty sleeping due to chronic nocturnal nasal congestion.
Methods
Subjects applied the strip at bedtime for 28 days (active phase; n = 70). Objective assessments included snoring variables, breathing route during sleep, and polysomnography measures compared with baseline. Nasal breathing, congestion, and sleep were measured subjectively using rating scales and questionnaires. During a crossover nasal resistance phase (n = 55), nasal resistance was measured using posterior rhinomanometry with the strip applied on one of two nights.
Results
In the active phase, breathing and sleep were perceived to improve, with less daytime sleepiness (P < 0.04) and increased ease of breathing, sleep quality, staying asleep, and feeling refreshed in the morning (all P < 0.0001). However, while objective polysomnography metrics were generally similar with and without the strip, median wake after sleep onset time was numerically reduced by ~ 11 min, and the spontaneous arousal rate fell by ~ 37%. In the nasal resistance phase (n = 55), median resistance (at 0.2–0.25 l/s) while asleep was 39.1% lower with (n = 37) versus without (n = 36) the strip (1.34 vs. 2.20 cmH2O/l/s; P = 0.048).
Conclusions
This exploratory study supports a role for the improved spring force nasal dilator strip in alleviating sleep-related symptoms in subjects with chronic nasal congestion, potentially via lowering nasal resistance and reducing nocturnal awakenings. A larger study is indicated to confirm these preliminary data.
ClinicalTrials.gov identifier
NCT03105297.
Funding
GlaxoSmithKline Consumer Healthcare.
Plain Language Summary
Plain language summary available for this article.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Plain language summary
Having a stuffy nose, or nasal congestion, may block or restrict airflow through the nose (higher nasal resistance), which is why breathing becomes more difficult with seasonal allergies or during a sinus infection. When congestion happens at night, individuals may develop sleep problems. In this study, subjects with ongoing nighttime nasal congestion used a strip placed on top of the nose (nasal strip) to see if opening the nasal passages would improve airflow (reduce nasal resistance), make breathing easier, and provide a better night’s sleep. Before and after using the nighttime nasal strip, subjects rated their breathing and sleep characteristics. Researchers measured subjects’ snoring, how they breathed during sleep (through the nose only or through both the mouth and the nose), and their sleep patterns.
The nasal strip improved the airflow through the nose (reduced nasal resistance) during sleep. Subjects who wore the nasal strip felt they could breathe more easily and had better sleep quality. Subjects also noted that after using the nasal strip they woke up feeling refreshed and were less sleepy during the day. There was no noticeable change in snoring with the nasal strip, but subjects who used the nasal strip tended to switch from mouth and nose breathing to nose breathing only during sleep. This nighttime nasal strip may help individuals with nasal congestion and difficulty sleeping to breathe and sleep better. This study provides a basis for future work exploring how breathing changes resulting from use of a nasal strip may influence sleep patterns.
Introduction
Nasal congestion is thought to be a leading symptom responsible for rhinitis-related sleep problems [1,2,3]. Nasal blockage interferes with the ability to fall asleep [4] and increases oral breathing during sleep [5]. Congestion is also a risk factor for snoring, unrefreshing sleep, obstructive sleep apnea, and excessive daytime sleepiness [4, 6, 7]. Increased nasal resistance is associated with snoring, upper airway resistance syndrome, and obstructive sleep apnea [1, 8,9,10].
The Breathe Right® Nasal Strip (BRNS; GSK Consumer Healthcare, Warren, NJ, USA) is a noninvasive, drug-free nasal dilator device that provides rapid relief of nasal congestion [11, 12]. Applied externally to the bridge of the nose, the adhesive strip gently opens the nasal passages by pulling out on the nasal vestibule, expanding the cross-sectional area of the nasal valve region [13]. Along with relieving subjective feelings of congestion/obstruction, the BRNS reduces nasal airway resistance and improves inspiratory airflow during nasal breathing [14,15,16,17,18,19]. Used during sleep, the original BRNS reduces the frequency, intensity, and volume of snoring [19,20,21,22]; improves sleep quality [20, 22, 23]; and reduces insomnia [23] and daytime sleepiness [20, 21].
A novel nasal dilator strip, asymmetrically shaped like a butterfly, was designed with improved spring forces compared with the original BRNS. Whereas the BRNS adheres to the nose flare and pulls out in a straight line across the nose, the asymmetric butterfly adheres to the cheek, enabling it to pull outward on multiple areas of the nose. This prototype is similar to the commercially marketed Breathe Right® Advanced strip (GSK Consumer Healthcare, Warren, NJ, USA). This exploratory study sought to characterize the performance of the butterfly-shaped prototype device in lowering nasal resistance during sleep and reducing signs, symptoms, and objective measures of sleep-disordered breathing in subjects with chronic nocturnal nasal congestion and difficulty sleeping due to their chronic nasal congestion.
Methods
Study Design and Procedures
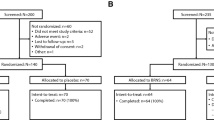
This was a baseline-controlled, stratified, 28-day study using a nasal dilator strip (active phase), followed by a two-night, randomized, crossover nasal resistance phase (ClinicalTrials.gov identifier: NCT03105297) conducted at Westmead Hospital, Westmead, New South Wales, Australia, over a 12-month period from September 2009 to August 2010. In-home measurement of the apnea-hypopnea index (AHI) was performed during the screening period using a portable polysomnography unit (Alice PDx, Philips Respironics, Amsterdam, The Netherlands) provided by the sleep laboratory. Subjects with an AHI ≥ 30 events/h were excluded. Demographics, medical history, and anthropomorphic data were collected at screening, 1–28 days before the baseline visit. At that time, subjects also provided ratings of nasal congestion, rhinorrhea, nasal itching, and sneezing (scale of none, mild, moderate, or severe). Subjects completed the Functional Outcomes of Sleep Questionnaire (FOSQ) [24], a 30-item, validated questionnaire that assesses the impact of excessive sleepiness on functional outcomes in five factors: activity level, vigilance, intimacy/sexual relationships, general productivity, and social outcome [25]; the Epworth Sleepiness Scale (ESS) [26]; and additional in-house questionnaires on sleep and snoring history. Acoustic rhinometry (Eccovision Acoustic Rhinometry System, Hood Laboratories, Pembroke, MA, USA) was performed at screening to measure the first two minimum cross-sectional areas and their corresponding nasal volumes in the anterior portion of the nose; distances of 0.5–3.0 cm into the nose were considered to represent the nasal valve region. Nasal endoscopy was performed to evaluate nasal septum, turbinates, meatuses, and nasal valve. The nasal vestibule wall elasticity method [27] was used to calculate the lateral nasal vestibule compliance (i.e., lateral nasal wall displacement measured on a linear scale per unit force, mm/g), derived by measuring the effect of the nasal dilator strip on nasal wall geometry. The investigator also assessed the cause of the nasal congestion. In-laboratory full polysomnography (PSG) was performed on day 1 to confirm baseline AHI and other sleep variables. Subjects were then stratified by AHI score: 0–5 events/h, > 5–15 events/h, or > 15 to < 30 events/h. All subjects were treated with the prototype nasal dilator strip. Subjects applied one strip at bedtime every night for 28 nights during the active phase. Study site personnel applied a strip to each subject on 1 of the 2 nights of the crossover nasal resistance phase. The strip was applied to the outer surface of the nose prior to bedtime as per the manufacturer’s instructions. See electronic supplementary materials for additional details on the study design and procedures.
Ethical Considerations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. This study was reviewed and approved by Western Sydney Local Health District Human Research Ethics Committee.
Study Population
Potentially eligible subjects were recruited by hospital staff from general medicine clinics, the sleep clinic, and the general population via advertising. Eligible subjects were ≥ 18 years of age, were in good general health, had leptorrhine noses (nasal tip protrusion index of ≥ 45) with chronic nasal congestion nightly or nearly nightly for at least the last year, and experienced trouble with sleep. See electronic supplementary materials for additional inclusion and exclusion criteria.
Study Outcomes
Objective Assessments
This study incorporated a number of objective assessments of snoring, sleep, and breathing during sleep. Snoring variables included number of snores per hour, percentage of sleep time spent snoring, and average and peak snore sound intensity. Breathing route during sleep was identified as nasal only or oronasal using a dual-channel thermistor. Sleep was assessed via PSG, and the following outcome variables recommended by the American Academy of Sleep Medicine [28] were measured: total sleep time, sleep onset latency, rapid eye movement (REM) latency, total non-REM sleep, total REM sleep, time awake after sleep onset, sleep efficiency, sleep architecture (percentage of time spent in each sleep stage: N1, N2, N3, and REM sleep) [28], arousals, and oxygen desaturations.
Nasal Airflow Resistance Measurement
Posterior rhinomanometry was used to measure the difference in transnasal pressure between the nasopharynx and external nares before and after nasal strip application while awake. Posterior rhinomanometry utilized a nasal continuous positive airway pressure (CPAP) mask to measure nasal airflow and pressure at the nares and a sealed mouthpiece to measure mouth pressure as a reflection of posterior oropharyngeal pressure [17]. During sleep in the nasal resistance phase, nasal resistance was measured using a mask, transnasal pressure [with a pressure catheter placed (without anesthesia) in the posterior nasopharynx via the same nasal passage on both nights and connected to a pressure transducer], and a lightweight flow meter [29]. The mask used for this assessment was a modified nasal CPAP mask that featured a wide base to avoid skin/nose pressure in the nares area, thereby minimizing any impact on nasal resistance and the effect of the nasal dilator strip. Nasal airflow and transnasal pressure difference (nasopharyngeal pressure referenced to nasal mask pressure) were recorded continuously on a Compumedics E Series PSG monitoring system (Compumedics Limited, Abbotsford, Victoria, Australia). See electronic supplementary materials for additional study outcomes.
Subjective Assessments
Subjects rated nasal airway breathing on two 100-mm visual analog scales (VAS) with anchors for “extremely difficult to breathe through my nose” and “extremely easy to breathe through my nose” for one scale and “nose is extremely blocked” and “nose is extremely clear” for the other scale. The VAS ratings were recorded before and after nasal dilator strip application, in both sitting and supine positions, at baseline and nights 8 and 29.
Subjects also rated their breathing after application of the nasal dilator strip using an 11-point numerical scale from − 5 (much worse) to 0 (same) to 5 (much better). This rating was made in the sitting and supine positions on nights 8 and 29.
On night 29, prior to sleep, subjects made global assessments of their experience with the nasal dilator strip compared with their sleep before study enrollment. For this assessment, subjects used a 5-point scale (− 2 = much worse, − 1 = somewhat worse, 0 = no change, 1 = somewhat improved, 2 = much improved) to rate each of the following: ease of breathing, staying asleep, falling back to sleep, waking up too early, number of awakenings, falling asleep, sleep quality, sleep depth, dry mouth upon awakening, morning headache, nocturia, and feeling refreshed in the morning. At night 29, subjects also repeated the ESS and FOSQ, results of which were compared with baseline.
Safety Outcomes
Treatment-emergent adverse events (TEAEs), defined as any untoward new conditions or exacerbation of existing conditions temporally associated with use of the nasal dilator strip, were graded for severity and assessed by the investigator as to relationship to treatment. Additional safety outcomes included serious TEAEs and incidents involving malfunction or deterioration in the characteristics and/or performance of the nasal dilator strip, or inadequacy in the labeling/instructions, that might have led to serious deterioration in health of the user.
Statistical Analyses
There were two analysis populations. The safety population consisted of all subjects who received the nasal dilator strips. The intent-to-treat (ITT) population consisted of all subjects who completed at least one objective measurement at both baseline and post-baseline.
Performance was based on change from baseline (before strip application) in objective and subjective sleep measures after 1 week and 4 weeks of nightly use of the nasal dilator strip and described using descriptive statistics. One-sample t tests were used to test for changes from baseline at the 5% significance level for acoustic rhinometry, rhinomanometry, PSG measures during the nasal resistance phase only, and subjective ratings and questionnaires. An alternative one-sample Wilcoxon signed-rank test was used in cases where the normal distribution assumptions for a t test were not met. Correlations between objective and subjective sleep measures were calculated using Pearson’s correlation coefficient and/or Spearman’s rank correlation coefficient.
A mixed model with repeated measures, with treatment and period as fixed effects, subjects as a random effect, and time as a repeated measures effect, was used to analyze nasal resistance measurements over the multiple time points recorded during the night; P values were calculated for comparisons of asleep supine measurements made for the night with the strip versus the night without the strip. PSG results during the nasal resistance phase were also analyzed with a mixed model, with treatment and period as fixed effects and subject as a random effect. Nasal airflow resistance was calculated using the power function \({\hbox{P}}_{\rm{TN}}=a{\hbox{V}}_{{\rm{TN}}^{b}}\), where PTN = transnasal pressure, VTN = nasal airflow, and a and b are constants and was fitted to transnasal pressure-flow curves by the method of least squares. See electronic supplementary materials for additional statistical information.
No imputations were made for missing data or for subjects who discontinued prematurely.
Results
Study Population
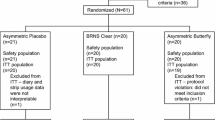
There were 103 subjects screened, of whom 91 had strip applications during the screening, baseline, and/or treatment phases (safety population). Seventy-one subjects had at least one baseline and postbaseline objective measurement (ITT population); one subject withdrew from the study, leaving 70 subjects who participated in the active phase and 55 in the nasal resistance phase (Fig. 1). Distribution in each stratum did not go as planned: of the 70 subjects enrolled, 52/70 (74%), 14/70 (20%), and 4/70 (6%) subjects were enrolled in the AHI 0–5 events/h, > 5–15 events/h, and > 15–< 30 events/h groups, respectively. Therefore, subgroup analyses by AHI status were limited because of the small number of subjects in the two higher strata.
The mean age of the ITT population was 48.5 years (range 20–77 years), and the majority of subjects were white (83%) and male (66%) (Table 1). Over 70% of subjects reported that they often or always snored; nearly 70% had never smoked, over 60% had never had a tonsillectomy or adenoidectomy, and almost all subjects did not take medicine to help them sleep (Table 1). The majority of subjects had high levels of respiratory sleep disturbance, with 73.0% (51/70) experiencing > 10 events/h on the Respiratory Disturbance Index (RDI). Nearly three-quarters of subjects (74%) reported moderate-to-severe nasal congestion, whereas other nasal symptoms were less common (sneezing, 25%; rhinorrhea, 19%; nasal itching, 15%). Nasal endoscopy revealed that 59% of subjects had nasal spurs, 48% had a moderately/severely deviated septum, 17% had moderate/severe turbinate enlargement, 13% had moderate/severe nasal valve collapse, and 11.6% had nasal polyps.
Effect of the Nasal Dilator on Nasal Area, Volume, and Compliance (Screening Phase)
Nasal cross-sectional area and volume significantly (P < 0.0001) increased by a mean of 0.59 cm (30%) and 0.90 cm3 (22%), respectively, after application of the nasal dilator strip, with subjects awake and seated. After the strip was applied, mean [standard deviation (SD)] nasal wall compliance was 0.12 (0.06) mm/g.
Primary Outcome: Nasal Resistance (Nasal Resistance Phase)
Nasal resistance generally increased when going from awake and seated to awake and supine to asleep and supine (Table 2). The nasal dilator strip reduced this increase in resistance. In the prespecified analysis of nasal resistance among those with nasal airflow rates of 0.2 l/s, median nasal airflow resistance was 21.7% lower while supine and asleep on the night with the strip (n = 22) compared with the night without the strip (n = 21); however, this difference was not statistically significant (P = 0.77). In the post hoc analysis of the larger subgroup of subjects who had nasal airflow rates of either 0.2 l/s or 0.25 l/s, median nasal airflow resistance while sleeping was 39.1% lower with versus without the strip, which was statistically significant (P = 0.048). Figure 2 shows mean nasal resistance over time from subjects who had at least one set of hourly data points for both observation nights (with and without the strip).
Group mean nasal resistance at flow rates of 0.2 and 0.25 l/s over time during the night, nasal resistance phase (ITT population) (n = 55a). aOnly 55 subjects entered the nasal resistance phase, and only subjects with data for both strip and no strip were used for the group means. ITT intent to treat, SD standard deviation
Other Objective Outcomes
There were no apparent changes in any snoring parameters (Table 3). There was a numerical trend suggesting a possible shift in breathing route from oronasal to nasal during both phases of the study (Table 3); however, statistical analysis of the data from the active phase by both Wilcoxon test (P = 0.359) and two-sample t test (P = 0.559) showed that this trend did not reach statistical significance.
PSG measures during the active phase are shown in Table 4. There were slight numerical trends toward a decrease in time awake after sleep onset, decrease in latency to REM sleep, increase in total REM sleep, and decrease in spontaneous arousals, likely attributable to the effect of the strip. Difference in wake after sleep onset did not reach statistical significance (Wilcoxon test P = 0.2751; two-sample t test P = 0.1364). (No statistical comparisons were made for the other outcomes.)
In the subgroup with AHI 0–5, the nasal dilator was associated with a reduction in the number of spontaneous arousals during the night, which decreased from a mean (SD) of 7.74 (4.11) at baseline to 5.81 (3.74) at night 8 and 6.15 (4.16) at night 29 of the active phase (P value not determined) and from 10.50 (7.22) on the night without the strip to 8.37 (6.47) on the night with the strip in the nasal resistance phase (P = 0.0235). The other AHI strata were not large enough to allow for any conclusions to be drawn.
During the nasal resistance phase, there were significantly fewer spontaneous arousals on the night with the strip compared with the night without the strip [mean (SD) 7.0 (6.2) vs. 8.7 (7.1) per hour, P = 0.014]. The other PSG outcomes showed no statistically significant differences on the night with the nasal dilator strip versus the night without the nasal dilator strip.
Subjective Measures (Active Phase Only)
The nasal dilator strip significantly improved VAS ratings for ease of breathing through the nose by 22.2% while sitting and 25.9% while supine, and how open the nose feels by 22.1% while sitting and 26.0% while supine (all P < 0.0001). On the 11-point scale from − 5 (much worse) to 5 (much better), mean (SD) ratings for ease of breathing after strip application were 3.2 (1.2) sitting and 3.1 (1.3) supine. All 12 global assessment items showed statistically significant improvements, particularly in subjective ratings of sleep-related symptoms, with the nasal strip compared with before the start of the study (Table 5).
During use of the nasal dilator strip, there was a statistically significant 10% improvement from baseline in daytime sleepiness on the ESS (Table 6). There were no significant changes from baseline on the FOSQ scale total score (Table 6) or on any of the individual subscales.
Safety Results
A total of 31 subjects reported 48 TEAEs, most of which were mild to moderate in severity. One severe headache and one instance of severe pain at the nose (reported by the same subject) were highly probably related to the study strip. The most common TEAEs were nasopharyngitis (nine events), application site reactions (six events), sore throat (five events), and headache (five events in four subjects). The application site reactions consisted of a mark on the nose followed by a sore nose and nose tender to the touch 3 days later in one subject, bruising across the bridge of the nose in one subject, and skin abrasion in one subject, all of which were considered highly probably related to treatment, and one subject had broken skin on the nose above the strip placement that was considered possibly related to treatment. All were mild and resolved during the study. There was one serious TEAE of moderate Bell’s palsy in a 50-year-old male with a benign history of herpes simplex virus infection more than 5 years prior to the study. The event, which was considered not related to the study treatment, occurred on day 12 of the active phase and resolved approximately 1 month after study completion. Three subjects discontinued treatment because of TEAEs: sunburn on the nose (n = 1), bronchitis (n = 1), and claustrophobia and difficulty breathing through the nose when the mask was in place during the nasal resistance phase of the study (n = 1).
Discussion
The question of whether the prototype nasal dilator strip can reduce nasal resistance during sleep in persons with chronic nocturnal nasal congestion requires evaluation in a larger study with greater statistical power. In this exploratory study, the prototype strip produced a 21.7% reduction in nasal resistance at a flow rate of 0.2 l/s during sleep at night compared with no strip in subjects with leptorrhine noses who had chronic nocturnal nasal congestion and trouble with sleep. However, only about 22 subjects contributed data at that flow rate, and in this small population, the difference did not reach statistical significance. We conducted a post hoc analysis of nasal resistance in those who achieved flow rates of 0.2 or 0.25 l/s and found that in this larger group (about 37 subjects) nasal resistance was decreased by 39.1% with versus without the strip, which was statistically significant (P < 0.05). However, we did not observe a sustained decrease in resistance throughout the night (Fig. 2). Numerous previous studies of the currently marketed BRNS have found it to reduce nasal resistance while awake and upright as well as during exercise [14,15,16,17, 19]. In addition, the prototype nasal dilator strip possibly countered the increase in nasal resistance that normally occurs when an individual goes from awake/seated to awake/supine to asleep/supine. A previous study has also shown an increase in resistance when transitioning from sitting to supine [30].
Numerical trends were observed suggesting that the strip may affect some objective (PSG) measures of sleep, which warrant investigation in a larger trial with sufficient power for statistical analysis. These slight trends included decreases in total time awake after sleep onset and latency to REM sleep, increases in total REM sleep, and fewer spontaneous arousals during the active phase. There were significantly fewer spontaneous arousals on the night with the strip versus the night without the strip (P = 0.014), but no other significant differences in PSG measures were observed during the resistance phase. Given that nasal dilator strips are designed to improve airflow during nasal breathing, it is unclear why the strip would reduce spontaneous arousals but not respiratory arousals or respiratory effort-related arousals; it is possible that the reduction in spontaneous arousals was a spurious finding or that it stemmed from better sleep quality in follow-up laboratory PSGs because of subject familiarity. Results of previous studies using the original BRNS have produced inconsistent PSG findings. One study conducted in persons with nasal obstruction and sleep-disordered breathing found that the BRNS significantly reduced the RDI (− 17%; P = 0.031) and the hypopnea index (− 2.8; P = 0.013) [31], whereas no improvements were seen in these outcomes in our study. One previous study found that the BRNS worsened some objective sleep measures on PSG, including sleep onset latency and some measures of sleep architecture [22]. Another PSG study conducted in habitual snorers with nasal obstruction found that the BRNS significantly improved sleep efficiency (total sleep time/time in bed—equivalent to reduced wake after sleep onset) but did not significantly affect other PSG measures including total sleep time, REM and non-REM sleep latency, sleep architecture, arousal index, or AHI compared with baseline or a placebo strip [19].
The lack of benefit with regard to snoring observed in this study contrasts with results of previous studies using the BRNS, which all showed a reduction in snoring frequency and/or intensity [19,20,21,22]. It is unknown whether these contrasting results are due to differences in the nasal dilator strips, differences in the study designs and populations, or differences in measurement techniques of snoring.
The nasal dilator strip used in this study led to a numerical increase in the proportion of subjects using nasal, instead of oronasal, breathing over the course of the night, but the difference did not reach statistical significance. A small but statistically significant reduction in daytime sleepiness was observed, as measured subjectively by a mean change of 0.85 on the ESS. Given the small magnitude of this change and the fact that mean ESS scores both at baseline (8.6) and after 28 days of using the nasal dilator strip (7.4) were within the high-normal daytime sleepiness range, the clinical relevance of this finding is unclear [32].
Subjective assessments of sleep showed significant improvements with the prototype nasal dilator strip (all P ≤ 0.05). These included improvements in global assessments of ability to fall and remain asleep as well as sleep depth and quality; reductions in nocturnal awakenings or awakening too early; and feeling more refreshed in the morning. Daytime sleepiness was reduced based on the ESS. These findings are consistent with those of a previous open-label study that reported significant improvements in subjective measures of sleep during use of the BRNS: in adults with a history of snoring, the BRNS reduced daytime sleepiness on the Stanford Sleepiness Scale (P < 0.01); improved sleep quality; reduced number of awakenings and sleepiness upon awakening; and improved morning concentration (all P < 0.05) [20]. However, while the global sleep assessments performed in the current study were all highly significant (P ≤ 0.002), there is an apparent lack of correlation between these subjective findings and many of the objective (PSG) measures of sleep, as no significant improvements were detected via PSG, including for arousals per hour, sleep efficiency, and sleep onset latency.
The prototype nasal dilator strip was well tolerated. Four (5.7%) of the 70 subjects experienced mild application site reactions.
The study population was limited to those with leptorrhine noses who had chronic nasal congestion nearly nightly for at least a year as well as difficulty sleeping and excluded subjects with anatomic nasal airway obstruction. Therefore, the findings may not be generalizable to all persons with nocturnal nasal congestion and sleep difficulties. Additional limitations are that the small sample size limited statistical power and little is known about what magnitude of change is clinically meaningful for many of the study outcomes.
Conclusions
This was a small, open-label, uncontrolled, exploratory study, so results are only hypothesis generating. The results suggest that the prototype BRNS improved some subjective nasal and sleep symptoms without exerting a meaningful impact on objective sleep metrics. The effect of the intervention on nasal resistance while awake or asleep remains unclear. Considering the established link among nasal congestion, increased nasal resistance, and disturbed sleep, potential benefits of the prototype nasal dilator strip on both objective and subjective sleep outcomes suggested in this preliminary study should be evaluated further in a larger study with greater statistical power to detect differences, not only in nasal resistance but also in PSG outcomes, to determine whether a reduction in resistance translates into any clinically meaningful improvements in sleep outcomes.
Change history
04 September 2019
The article “Objective and Subjective Effects of a Prototype Nasal Dilator Strip on Sleep in Subjects with Chronic Nocturnal Nasal Congestion”, written by John R. Wheatley, Terence C. Amis, Sharon A. Lee, Renee Ciesla, Gilbert Shanga was originally published electronically on the publisher’s internet portal (currently SpringerLink) on May, 22, 2019 without Open Access. The article has now been made Open Access.
References
Storms W. Allergic rhinitis-induced nasal congestion: its impact on sleep quality. Prim Care Respir J. 2008;17(1):7–18.
Craig TJ, Teets S, Lehman EB, Chinchilli VM, Zwillich C. Nasal congestion secondary to allergic rhinitis as a cause of sleep disturbance and daytime fatigue and the response to topical nasal corticosteroids. J Allergy Clin Immunol. 1998;101(5):633–7.
Stull DE, Roberts L, Frank L, Heithoff K. Relationship of nasal congestion with sleep, mood, and productivity. Curr Med Res Opin. 2007;23(4):811–9.
Hussain SF, Cloonan YK, Rahbar MH, Islam M. Association of self-reported nasal blockage with sleep-disordered breathing and excessive daytime sleepiness in Pakistani employed adults. Sleep Breath = Schlaf & Atmung. 2010;14(4):345–51.
Suzuki M, Furukawa T, Sugimoto A, Katada K, Kotani R, Yoshizawa T. Relationship between oral flow patterns, nasal obstruction, and respiratory events during sleep. J Clin Sleep Med. 2015;11(8):855–60.
Young T, Finn L, Palta M. Chronic nasal congestion at night is a risk factor for snoring in a population-based cohort study. Arch Intern Med. 2001;161(12):1514–9.
Lofaso F, Coste A, d’Ortho MP, Zerah-Lancner F, Delclaux C, Goldenberg F, et al. Nasal obstruction as a risk factor for sleep apnoea syndrome. Eur Respir J. 2000;16(4):639–43.
Li HY, Wang PC, Hsu CY, Cheng ML, Liou CC, Chen NH. Nasal resistance in patients with obstructive sleep apnea. ORL J Otorhinolaryngol Relat Spec. 2005;67(2):70–4.
Metes A, Ohki M, Cole P, Haight JS, Hoffstein V. Snoring, apnea and nasal resistance in men and women. J Otolaryngol. 1991;20(1):57–61.
McNicholas WT, Tarlo S, Cole P, Zamel N, Rutherford R, Griffin D, et al. Obstructive apneas during sleep in patients with seasonal allergic rhinitis. Am Rev Respir Dis. 1982;126(4):625–8.
Hoyvoll LR, Lunde K, Li HS, Dahle S, Wentzel-Larsen T, Steinsvag SK. Effects of an external nasal dilator strip (ENDS) compared to xylometazolin nasal spray. Eur Arch Otorhinolaryngol. 2007;264(11):1289–94.
Latte J, Taverner D. Opening the nasal valve with external dilators reduces congestive symptoms in normal subjects. Am J Rhinol. 2005;19(2):215–9.
Kiyohara N, Badger C, Tjoa T, Wong B. A comparison of over-the-counter mechanical nasal dilators: a systematic review. JAMA Facial Plast Surg. 2016;18(5):385–9.
Roithmann R, Chapnik J, Cole P, Szalai J, Zamel N. Role of the external nasal dilator in the management of nasal obstruction. Laryngoscope. 1998;108(5):712–5.
Gehring JM, Garlick SR, Wheatley JR, Amis TC. Nasal resistance and flow resistive work of nasal breathing during exercise: effects of a nasal dilator strip. J Appl Physiol (1985). 2000;89(3):1114–22.
Seto-Poon M, Amis TC, Kirkness JP, Wheatley JR. Nasal dilator strips delay the onset of oral route breathing during exercise. Can J Appl Physiol. 1999;24(6):538–47.
Kirkness JP, Wheatley JR, Amis TC. Nasal airflow dynamics: mechanisms and responses associated with an external nasal dilator strip. Eur Respir J. 2000;15(5):929–36.
Di Somma EM, West SN, Wheatley JR, Amis TC. Nasal dilator strips increase maximum inspiratory flow via nasal wall stabilization. Laryngoscope. 1999;109(5):780–4.
Pevernagie D, Hamans E, Van Cauwenberge P, Pauwels R. External nasal dilation reduces snoring in chronic rhinitis patients: a randomized controlled trial. Eur Respir J. 2000;15(6):996–1000.
Scharf MB, Brannen DE, McDannold M. A subjective evaluation of a nasal dilator on sleep & snoring. Ear Nose Throat J. 1994;73(6):395–401.
Ulfberg J, Fenton G. Effect of Breathe Right nasal strip on snoring. Rhinology. 1997;35(2):50–2.
Todorova A, Schellenberg R, Hofmann HC, Dimpfel W. Effect of the external nasal dilator Breathe Right on snoring. Eur J Med Res. 1998;3(8):367–79.
Krakow B, Melendrez D, Sisley B, Warner TD, Krakow J, Leahigh L, et al. Nasal dilator strip therapy for chronic sleep-maintenance insomnia and symptoms of sleep-disordered breathing: a randomized controlled trial. Sleep Breath = Schlaf & Atmung. 2006;10(1):16–28.
Weaver TE, Laizner AM, Evans LK, Maislin G, Chugh DK, Lyon K, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20(10):835–43.
Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–91.
Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–5.
Amis TC, Kirkness JP, di Somma E, Wheatley JR. Nasal vestibule wall elasticity: interactions with a nasal dilator strip. J Appl Physiol (1985). 1999;86(5):1638–43.
Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for scoring of sleep and associated events: rules, terminology and technical specifications. 1st ed. Westchester: American Academy of Sleep Medicine; 2007.
Kohler M, Thurnheer R, Bloch KE. Side-selective, unobtrusive monitoring of nasal airflow and conductance. J Appl Physiol (1985). 2006;101(6):1760–5.
Desfonds P, Planes C, Fuhrman C, Foucher A, Raffestin B. Nasal resistance in snorers with or without sleep apnea: effect of posture and nasal ventilation with continuous positive airway pressure. Sleep. 1998;21(6):625–32.
Gosepath J, Amedee RG, Romantschuck S, Mann WJ. Breathe Right nasal strips and the respiratory disturbance index in sleep related breathing disorders. Am J Rhinol. 1999;13(5):385–9.
Johns MW. About the Epworth Sleepiness Scale. 2018. http://epworthsleepinessscale.com/about-the-ess/. Accessed 27 June 2018.
Acknowledgements
Funding
Funding for this study and article processing charges was provided by GlaxoSmithKline Consumer Healthcare, Warren, NJ, USA. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. The authors thank the participants of the study.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Medical Writing, Editorial, and Other Assistance
Medical writing and editorial assistance was provided by Lauren Cerruto and Diane M. Sloan, PharmD, at Peloton Advantage, LLC, an OPEN Health Company, Parsippany, NJ, USA, and was funded by GlaxoSmithKline Consumer Healthcare. The authors would like to thank Ms. Manisha Verma, Ms. Melanie Madronio, Mr. Stephen Lambert, Ms. Warde Elias, Ms. Rita Ginn, Ms. Monica Baarbe, Ms. Angela Wong, and Ms. Andrea Vidal for assistance with data collection and analysis and also thank the staff from the Westmead Hospital Sleep Disorders Laboratory.
Disclosures
John R. Wheatley has received funding for this research project from GlaxoSmithKline Consumer Healthcare and has served on an advisory board for Novartis. Terence C. Amis and Sharon A. Lee have nothing to declare. Renee Ciesla is an employee of GlaxoSmithKline Warren, NJ, USA. Gilbert Shanga is an employee of GlaxoSmithKline Warren, NJ, USA.
Compliance with Ethics and Guidelines
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. This study was reviewed and approved by Western Sydney Local Health District Human Research Ethics Committee.
Data Availability
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Author information
Authors and Affiliations
Corresponding author
Additional information
The original version of this article was revised due to retrospective open access.
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.8063564.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wheatley, J.R., Amis, T.C., Lee, S.A. et al. Objective and Subjective Effects of a Prototype Nasal Dilator Strip on Sleep in Subjects with Chronic Nocturnal Nasal Congestion. Adv Ther 36, 1657–1671 (2019). https://doi.org/10.1007/s12325-019-00980-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-019-00980-z