Abstract
Emerging anti-vascular endothelial growth factor (anti-VEGF) therapies for neovascular age-related macular degeneration (nAMD) have revolutionised medical retina practice and the management and eventual outcome of nAMD. Recent research has focused on evaluating and comparing the efficacy of the two most widely employed anti-VEGF agents, bevacizumab and ranibizumab; however, a subgroup of patients with nAMD demonstrates a suboptimal response to standard therapy. We have therefore conducted a review of pertinent studies published until August 2018 which have documented the clinical efficacy when switching to a different anti-VEGF. Evidence on baseline disease characteristics, injection frequency and disease outcome has been obtained for patients treated with ranibizumab 0.5 mg and/or bevacizumab 1.25 mg and were switched to aflibercept 2 mg. Our review identified 45 studies investigating switching to aflibercept. Our review showed a clear anatomical benefit after the switch in terms of central retinal thickness and pigment epithelium detachment characteristics, whereas the functional outcomes were variable. Remarkable heterogeneity was documented among the relevant studies with regard to several factors including the baseline characteristics of the cohorts, the non-response definition and previous treatment protocols. Larger prospective trials with appropriate control arms are therefore required to elucidate the potential benefit when switching between anti-VEGF agents in refractory nAMD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Age-related macular degeneration (AMD) is a leading cause of irreversible blindness in the population over the age of 50 years in the developed world [1, 2]. The exudative, neovascular form of the disease usually results in rapid loss of vision [3]. The intravitreal use of recombinant, humanized, monoclonal antibodies Fab to neutralize all active forms of vascular endothelial growth factor A (VEGF-A) such as ranibizumab (Lucentis; Genetech, San Francisco, CA; and Novartis, West Sussex, UK) and bevacizumab (Avastin; Roche and Genentech, Basel, Switzerland) revolutionised AMD therapy and has provided significant benefits with respect to the anatomic and visual acuity (VA) outcomes as demonstrated in the MARINA and ANCHOR studies [4, 5]. A further 2008 Cochrane systematic review concluded that ranibizumab therapy was beneficial for the treatment of AMD and associated with relatively few adverse effects [6]. The ANCHOR and MARINA trials demonstrated that between 94.3% and 94.6% of patients treated with ranibizumab maintained vision at 12 months (loss of fewer than 15 letters) compared to 62.2% on placebo. Moreover, visual acuity improved by more than 15 letters in 24.8–40.3% of patients who received ranibizumab compared to only 5.0% in the placebo control group [4, 5].
Although this represents a satisfactory treatment response, approximately 5% of this group experienced significant deterioration in visual acuity (loss of more than 15 letters at 12 months) [4, 5]. The percentage of participants who experienced deterioration in visual acuity increased to approximately 10% at 24 months [7]. The CATT study reported similar visual acuity outcomes after 1 and 2 years for ranibizumab and bevacizumab under the same treatment protocol [8]. Importantly, persistent macular fluid was detected by optical coherence tomography (OCT) in 51.5% of the patients treated with monthly ranibizumab and 67.4% of those treated with monthly bevacizumab injections after 2 years. These rates were even higher when the pro re nata (PRN) protocol was used [8]. In addition, a reduction in visual acuity of more than 15 ETDRS (Early Treatment Diabetic Retinopathy Study) letters at 2 years was reported in 6.7% and 7.2% of patients treated with monthly and PRN ranibizumab respectively. The respective percentages for the bevacizumab group were 7.8% (monthly) and 11.6% (PRN) [8].
Several studies have investigated the predictive value of clinical and genetic factors in the treatment response. A poorer treatment outcome has been associated with greater age, better baseline VA, larger choroidal neovascularisation (CNV) lesion at baseline and a greater interval between onset of symptoms and initiation of treatment [9]. Moreover, extensive research has been performed to identify genetic associations with response to anti-VEGF therapies but the results are as yet inconclusive [10].
The subgroup showing resistant or refractory AMD is expected to incur significant morbidity and therefore specific research of alternative therapeutic regimens to improve outcomes is warranted. In addition to significant morbidity, AMD bears significant socioeconomic implications through direct and indirect medical and social cost [11, 12], loss of earnings, loss of healthy life [13] and a significant reduction in vision-related quality of life (VRQoL) [14, 15]. Conversely, a subsequent improvement in visual acuity is shown to be associated with improved functioning and quality of life [16].
A number of studies investigating the therapeutic potential of aflibercept, a recombinant soluble decoy receptor fusion protein with a greater affinity for VEGF-A, VEGF-B and placental growth factor [17], have been conducted. VIEW 1 and VIEW 2 multicentre studies assessed the efficacy and safety of aflibercept [18]. Various aflibercept treatment regimens have been used to optimise the AMD treatment and more recently some studies have investigated the potential of this therapy specifically in recalcitrant nAMD. The aim of this article was to critically review the success of switching from the other two antiangiogenic agents to aflibercept in individuals with refractory or recurrent AMD.
Methods
We searched Cochrane Library and MEDLINE for publications related to the review objective. Our search strategy consisted of subject headings and keywords ‘wet macular degeneration’, ‘angiogenesis inhibitors’, ‘aflibercept’, ‘VEGF trap’, ‘bevacizumab’, ‘ranibizumab’, ‘switch’, ‘refractory’, ‘resistant’, ‘transition’ and ‘recalcitrant’. Databases were last searched in August 2018.
Search results were analysed by title and abstract to determine their relevance to the review objective. Publications were included in the analysis if participants had a diagnosis of wet, neovascular or exudative AMD, previously treated with intravitreal injections of 0.5 mg ranibizumab, 1.25 mg bevacizumab or both, who were switched to treatment with aflibercept because of persistent intraretinal or subretinal fluid as determined by OCT. Exclusion criteria included a sample size of smaller than ten eyes and a follow-up period of less than 6 months. Only studies published in English were included.
Data collected from the publications included date of publication, study design, number of participants, inclusion/exclusion criteria of the study, intervention protocol, mean injection frequency, follow-up period, visual acuity and anatomical outcomes. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Results
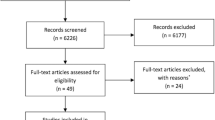
Our search yielded 66 studies relevant to our review objective, published between July 2013 and August 2018. Twenty-one publications were excluded from our analysis: 5 publications were review articles, 3 publications had a small sample size and 13 publications did not have a sufficient follow-up period. The remaining 45 publications were included in our data analysis. A summary of the results of our search and data collection is shown in Tables 1 and 2.
Fifteen out of the 45 included studies were prospective; three of them were multicentre single-arm and one of them was a single-centre randomised with control arm. The remaining 30 were retrospective studies, one of which was a multicentre electronic medical record (EMR) review with control arm.
The inclusion and exclusion criteria for participants of the 45 studies displayed a significant degree of heterogeneity. Studies lacked a clear consensus regarding baseline participant features such as participant age, baseline visual acuity, persistent or refractory subretinal fluid, bilateral or unilateral AMD, previous intervention frequency and duration and the presence or absence of additional retinal pathology including pigment epithelial detachment (PED), subretinal fibrosis, geographic atrophy, idiopathic polypoidal choroidal vasculopathy, central serous retinopathy or cystic degeneration. In most studies performed to date refractory status was defined by the presence of intra- or subretinal fluid (IRF, SRF) and/or pigment epithelium detachment (PED), with or without macular haemorrhage despite regular treatment. The switch to aflibercept was carried out after 3–9 anti-VEGF (bevacizumab and/or ranibizumab) intravitreal injections, which were performed within a period of 3–12 months. The intervals between the last anti-VEGF injection and the first aflibercept injection was no longer than 6 weeks.
Prospective Studies
Several clinical trials have assessed the efficacy of switching to aflibercept in nAMD patients following unsatisfactory response to prior therapy (Table 1). In a multicentre prospective study, Tiosano et al. [19] studied 47 eyes from 46 nAMD patients with incomplete response to bevacizumab. Twenty-eight weeks after switching to aflibercept therapy, the mean best corrected visual acuity (BCVA) showed mild but statistical significant improvement from 60.3 to 63.1 EDTRS letters (p = 0.02), while the central subfield thickness (CST) was significantly reduced from 409 to 277 µm (p = 0.0002) [19]. When switching patients from ranibizumab to aflibercept, Aghdam et al. [20] found a significant mean BCVA increase from 45 to 59 EDTRS letters (p < 0.001) after 12 months follow-up in 22 eyes (19 patients) with nAMD. The CST was also significantly reduced from 400 to 304 µm (p = 0.003). Similarly, Kawashima et al. [21], Chang et al. [22], Singh et al. [23], Curry et al. [24], Blanco-Garavito et al. [25] and Zhu et al. [26] have demonstrated a statistically significant improvement in both BCVA and CRT following switching from ranibizumab and/or bevacizumab to aflibercept for the treatment of refractory nAMD.
In contrast, a number of studies have failed to record a significant improvement in terms of visual acuity when switching to aflibercept therapy despite identifying a significant improvement in terms of central retinal thickness (CRT). More specifically, Wykoff et al. [27] prospectively studied patients with recalcitrant nAMD initially treated with ranibizumab. Six months after switching to aflibercept therapy, there was a significant reduction of 23.6 µm in the CST (p = 0.018) but BCVA did not improve [27]. No significant gain in BCVA has been reported in four other studies, despite the significant CRT reduction [28,29,30,31].
It is worth noting that to date the only prospective randomised clinical trial with a control arm was conducted by Mantel et al. [32]. This small study included 21 eyes (19 patients) that had been treated with ranibizumab and still required monthly injections at the end of the second year of treatment. These patients were randomised to either continue ranibizumab injections or switch to aflibercept. After 12 months, the BCVA change was not found to be significantly different between the two groups. In addition, the mean retreatment interval was 1.13 months in the aflibercept group and 1.14 months in the control group [32].
Jorstad et al. [33] evaluated prospectively the efficacy of switching from bevacizumab or ranibizumab to aflibercept in 50 eyes from 47 nAMD patients with persistent macular fluid. Notably, these authors reported a statistically significant reduction in BCVA after 2 years of follow-up, from 0.25 to 0.32 logMAR (p = 0.005), even though no significant difference was detected during the first year (0.24 logMAR) [33].
Retrospective Evidence
A large number of retrospective studies have evaluated the outcomes of transition to aflibercept therapy in nAMD (Table 2). Chan et al. [34] included 189 cases, the majority of which (82%) were refractory to bevacizumab and ranibizumab injections. They documented a significant improvement in BCVA of 0.081 logMAR (p < 0.001) and a significant reduction in CST of 24.9 μm (p < 0.001) after 6 months [34]. Further statistically significant improvements of BCVA and CRT were also reported in other studies [35,36,37,38]. Narayan et al. [39] found a significant BCVA improvement in patients with refractory to ranibizumab therapy but with no CRT data.
In contrast, several clinical trials have reported a lack of BCVA improvement despite significantly improved anatomical outcomes [40,41,42,43,44,45,46,47,48,49,50,51,52,53]. Chatziralli et al. [54] reported the results of large retrospective study which included 447 eyes with persistent nAMD despite treatment with ranibizumab injections. Twelve months after switching to aflibercept, the BCVA did not change (from 63.7 to 63.3 ETDRS letters), although the CST was significantly reduced from 271 to 242 μm (p < 0.001) [54]. Moreover, a non-significant VA change without any CRT data was reported by Kanesa-Thasan et al. [55], Barthelmes et al. [56] and Tyagi et al. [57].
Homer et al. [58] reported an unchanged BCVA (from 0.42 to 0.42 logMAR) and a small, non-significant reduction of CST (from 292 to 283 μm) in a small cohort of 21 eyes with persistent exudation 2 years after converting to aflibercept. Similarly, Ferrone et al. [59] and Moon et al. [60] reported non-significant functional and anatomical changes 6 months after the switch.
It is worth noting that a single study reported a significant reduction in terms of BCVA when switching from ranibizumab and/or bevacizumab to aflibercept [61]. This study demonstrated a significant BCVA reduction, from 56.5 to 50.3 letters (p < 0.001), 3 years after transition to aflibercept in 164 nAMD eyes, although vision was found to be stable 1 year after the switch. Interestingly, CRT was significantly improved at both time points [61] so one could hypothesize that other factors and the natural course of the disease may account for the ultimate reduction in vision after transition to aflibercept.
The only retrospective study with a control arm was reported by Lee et al. [62]. It was a multicentre study comparing the outcomes of 448 eyes that were switched to aflibercept after 6 monthly ranibizumab injections as opposed to 896 eyes which continued ranibizumab therapy. Despite an initial improvement in BCVA after switching to aflibercept therapy, this benefit was not detected 6 months after the switch [62].
Pigment Epithelial Detachment (PED)
In terms of PED characteristics, such as PED height and volume, several studies showed significant improvement when switching to aflibercept [21, 25, 28, 34, 38, 42, 52, 55]. In contrast, Kim et al. [31] reported a stable PED volume 12 months after conversion to aflibercept in cases with refractory PED. Tyagi et al. [57] found a significant reduction in PED height but not PED width in 50 cases, 12 months after switching from ranibizumab to aflibercept.
Number of Injections
With regard to the number of injections, several studies have shown a significant reduction in the number of injections after a therapeutic switch to aflibercept [29, 41, 45, 56, 58], while others have reported a non-significant change in the overall number of injections [32, 44, 53]. Sarao et al. [29] also found an improvement in the mean injection interval, from 5.3 to 13.6 weeks, in a study of 50 cases that were switched from ranibizumab to aflibercept and were followed up for 1 year.
Quality of Life
As for the impact of switching intravitreal therapies on quality of life, Zhu et al. [26] prospectively assessed the changes in vision-related quality of life in 49 patients with refractory nAMD with the use of the National Eye Institute Visual Functioning Questionnaire 25 (NEI VFQ-25). The NEI VFQ-25 composite scores improved significantly after 1 year and this improvement correlated with the significant changes in BCVA but not CMT [26].
Discussion
Various contributing factors have been implicated in the suboptimal response to anti-VEGF treatment for patients suffering from exudative AMD [9, 10]. The hypothesis of reduced anti-VEGF efficacy with repeated injections or a form of treatment-related tachyphylaxis may apply and has resulted in switching between anti-VEGF treatments as a logical management step in clinical practice [63]. Amoaku et al. [63] proposed a grading system for the response of patients with exudative AMD to anti-VEGF treatment based on functional and morphological criteria. The response was categorized as good, partial poor and no response in terms of visual acuity and OCT parameters including subretinal/intraretinal fluid, pigment epithelial detachment and central retinal thickness. The authors suggested that switching between anti-VEGF agents is justified only when there is evidence of poor or inadequate response in either function or morphology [63].
Several conclusions regarding the success of switching from ranibizumab or bevacizumab to aflibercept in patients with exudative AMD can be drawn from our review. In terms of visual acuity many studies suggest a meaningful improvement after the switch. However, the only two comparative studies that have been conducted failed to identify a significant visual difference following treatment change as opposed to continuing with the same agent (ranibizumab) [32, 62]. In addition, a large retrospective study by Lee et al. [62] demonstrated a significant improvement in terms of VA immediately after switching to aflibercept; however, this improvement was not sustained after 6 months. The authors suggest that this result is due to tachyphylaxis to ranibizumab rather than to superiority of aflibercept. Moreover, Mantel et al. [32] showed a non-significant deterioration in VA in patients that switched to aflibercept when compared to controls. Long-term visual outcomes do not appear to be more favourable either [33, 61] but there may be many compounding factors beyond 2 years of follow-up. Jorstad et al. [33] and Cardoso et al. [61] found a significant deterioration in VA at 24 and 36 months respectively after switching to aflibercept, despite stability in their 12-month results [33, 61]. Since there was no control group in either study, the result can probably be attributed to the natural course of the disease rather than to the actual switch to aflibercept.
In contrast, the majority of switch studies show a significant improvement in anatomical outcomes immediately after the switch that has been maintained throughout the follow-up period [19, 21,22,23,24,25,26,27,28,29, 31, 33,34,35,36, 38, 40,41,42,43,44,45,46,47,48, 50,51,52,53,54,55, 59,60,61, 64, 65]. Anatomical features including CRT and PED measured by OCT are consistently improved to a significant degree in almost all of the reviewed studies.
In addition, the injection interval appears to be increased or at least remained stable after the switch in all the pertinent studies. Moreover, the only study that assessed the quality of life showed significant improvement as an additional benefit of the therapeutic switch to aflibercept [26].
Overall most of the studies support the concept of switching to aflibercept in those patients that show an insufficient response to the other anti-VEGF agents based on improved anatomical outcomes, reduction in intraretinal fluid, subretinal fluid, PED and potentially extended injection intervals. Various studies have shown improvement or stabilization of visual outcomes when switching to aflibercept after suboptimal response to the other two anti-VEGF agents. Nevertheless, it should be emphasized that nonrandomised, retrospective case series on switching are difficult and challenging to interpret because of the plethora of technical biases, lack of controls and the presence of confounding factors. Furthermore, the methodological heterogeneity of the studies creates much uncertainty when trying to answer a specific clinical question such as the one dealt with here. However, case series have a place in preliminary investigational research and are informative in understanding potential strategies for optimising patient care.
Furthermore, the lack of subgroup analysis data, AMD phenotype classification and uniform design of the studies makes any interpretation of benefits a challenging task. The absence of comparative arm in most published studies to date makes interpretation of the findings difficult. Regrettably, the two studies with control arms have been either retrospective or underpowered because of a small sample size [32, 62]. The absence of masking in the interpretation of fundus fluorescein angiograms and optical coherence tomography examinations is also a methodological setback. Moreover, registration of changes in refractive errors and documentation of cataract were absent in most of the studies performed to date. Additionally, overall one has to consider whether optimal timely and sufficient treatment has been provided to patients with refractive AMD and exclude all possible causes of non-response including masking syndromes.
Conclusions
The present review attempted to critically summarise current evidence and success rate when switching from ranibizumab or bevacizumab to aflibercept patients with exudative AMD. Although there is a significant body of literature reporting variable results, the cumulative evidence suggests that there may be a benefit. Patients with exudative AMD refractory to other anti-VEGF agents may gain substantial benefit from switching to aflibercept in terms of anatomical outcome and interval between injections; however, there is still uncertainty regarding the visual outcome. Adequately powered randomised controlled trials with appropriate controls are required to address the real benefits of the switch between anti-VEGF agents and establish treatment guidelines for better anatomical and functional outcome.
References
Bressler N. Age-related macular degeneration is the leading cause of blindness. JAMA. 2004;291(15):1900–1.
Friedman D, O’Colmain B, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–72.
Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379(9827):1728–38.
Rosenfeld P, Brown D, Heier J, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31.
Brown D, Kaiser P, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;335:1432–44.
Vedula SS, Krzystolik MG. Antiangiogenic therapy with anti-vascular endothelial growth factor modalities for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2008;2:CD005139.
Rosenfeld PJ, Shapiro H, Tuomi L, et al. Characteristics of patients losing vision after 2 years of monthly dosing in the phase III ranibizumab clinical trials. Ophthalmology. 2011;118(3):523–30.
Martin DF, Maguire MG, Fine SL, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results: comparison of age-related macular degeneration treatments trials research group. Ophthalmology. 2012;119(7):1388–98.
Zhang X, Lai TYY. Baseline predictors of visual acuity outcome in patients with wet age-related macular degeneration. Biomed Res Int. 2018;2018:9640131.
Lores-Motta L, de Jong EK, den Hollander AI. Exploring the use of molecular biomarkers for precision medicine in age-related macular degeneration. Mol Diagn Ther. 2018;22(3):315–43.
Rein D, Zhang P, Wirth K, et al. The economic burden of major adult visual disorders in the United States. Arch Ophthalmol. 2006;124:1754–60.
Husler S, Schmid H. Coping with wet age-related macular degeneration: a study from Switzerland. Klin Monbl Augenheilkd. 2013;230(12):1251–6.
Taylor HR, Pezzullo ML, Keeffe JE. The economic impact and cost of visual impairment in Australia. Br J Ophthalmol. 2006;90(3):272–5.
Lin JC, Yu JH. Assessment of quality of life among Taiwanese patients with visual impairment. J Formos Med Assoc. 2012;111(10):572–9.
Brown G, Sharma S, Brown M, Kistler J. Utility values and age-related macular degeneration. Arch Ophthalmol. 2000;118(1):47–51.
Bressler NM, Chang TS, Suner IJ, et al. Vision-related function after ranibizumab treatment by better- or worse-seeing eye: clinical trial results from MARINA and ANCHOR. Ophthalmology. 2010;117(4):747–56.
Hanout M, Ferraz D, Ansari M, et al. Therapies for neovascular age-related macular degeneration: current approaches and pharmacologic agents in development. Biomed Res Int. 2013;2013:830837.
Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121(1):193–201.
Tiosano L, Segal O, Mathalone N, et al. Aflibercept as a second line therapy for neovascular age related macular degeneration in Israel (ASLI) study. Eye (Lond). 2017;31(6):890–8.
Aghdam KA, Pielen A, Framme C, Junker B. Visual and anatomic outcomes after conversion to aflibercept in neovascular age-related macular degeneration: 12-month results. Eur J Ophthalmol. 2016;26(5):473–8.
Kawashima Y, Oishi A, Tsujikawa A, et al. Effects of aflibercept for ranibizumab-resistant neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. 2014;253(9):1471–7.
Chang AA, Broadhead GK, Hong T, et al. Intravitreal aflibercept for treatment-resistant neovascular age-related macular degeneration: 12-month safety and efficacy outcomes. Ophthalmic Res. 2015;55(2):84–90.
Singh RP, Srivastava SK, Ehlers JP, et al. A single-arm, investigator-initiated study of the efficacy, safety, and tolerability of intravitreal aflibercept injection in subjects with exudative age-related macular degeneration previously treated with ranibizumab or bevacizumab (ASSESS study): 12-month analysis. Clin Ophthalmol. 2015;9:1759–66.
Curry B, Bylsma G, Hewitt AW, Verma N. The VEGF treatment of AMD switch study (The vTAS Study). Asia Pac J Ophthalmol (Phila). 2017;6(6):481–7.
Blanco-Garavito R, Jung C, Uzzan J, et al. Aflibercept after ranibiumab intravitreal injections in exudative age-related macular degeneration: the ARI2 Study. Retina. 2017;00:1–8.
Zhu M, Wijeyakumar W, Syed AR, et al. Vision-related quality of life: 12-month aflibercept treatment in patients with treatment-resistant neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2017;255(3):475–84.
Wykoff CC, Brown DM, Maldonado ME, Croft DE. Aflibercept treatment for patients with exudative age-related macular degeneration who were incomplete responders to multiple ranibizumab injections (TURF trial). Br J Ophthalmol. 2014;98(7):951–5.
Grewal DS, Gill MK, Sarezky D, Lyon AT, Mirza RG. Visual and anatomical outcomes following intravitreal aflibercept in eyes with recalcitrant neovascular age-related macular degeneration: 12-month results. Eye (Lond). 2014;28(7):895–9.
Sarao V, Parravano M, Veritti D, Arias L, Varano M, Lanzetta P. Intravitreal aflibercept for choroidal neovascularization due to age-related macular degeneration unresponsive to ranibiumab therapy. Retina. 2016;36:770–7.
Sleiman A. Aflibercept in refractory wet AMD treated with ranibizumab: anatomical and visual outcome. Saudi J Ophthalmol. 2016;30(4):227–32.
Kim K, Kim E, Kim Y, Yang J, Yu S, Kwak H. Outcome of intravitreal aflibercept for refractory pigment epithelial detachment with or without subretinal fluid and secondary to age-related macular degeneration. Retina. 2017;1:1–11.
Mantel I, Gianniou C, Dirani A. Conversion to aflibercept therapy versus continuing with ranibizumab therapy for neovascular age-related macular degeneration dependent on monthly ranibizumab treatment. Retina. 2016;6:53–8.
Jorstad OK, Faber RT, Moe MC. Two-year functional and anatomical results after converting treatment resistant eyes with exudative age-related macular degeneration to aflibercept in accordance with a treat and extend protocol. Acta Ophthalmol. 2017;95(5):460–3.
Chan C, Jain A, Sadda S, Varshney N. Optical coherence tomographic and visual results at six months after transitioning to aflibercept for patients on prior ranibizumab or bevacizumab treatment for exudative age-related macular degeneration (an American Ophthlmological Society thesis). Trans Am Ophthalmol Soc. 2014;112:160–98.
Kumar N, Marsiglia M, Mrejen S, et al. Visual and anatomical outcomes of intravitreal aflibercept in eyes with persistent subfoveal fluid despite previous treatments with ranbizumab in patients with neovascular age-related macular degeneration. Retina. 2013;33:1605–12.
Hirakata T, Fujinami K, Watanabe K, Sasaki M, Noda T, Akiyama K. One-year outcome of intravitreal aflibercept injection for age-related macular degeneration resistant to ranibizumab: rapid morphologic recovery and subsequent visual improvement. Clin Ophthalmol. 2016;10:969–77.
Warwick AN, Leaver HH, Lotery AJ, Goverdhan SV. Fixed bimonthly aflibercept in naive and switched neovascular age-related macular degeneration patients: 1 year outcomes. Int J Ophthalmol. 2016;9(8):1156–62.
Kocak I. Intravitreal aflibercept in treatment-resistant pigment epithelial detachment. Int Ophthalmol. 2017;37(3):531–7.
Narayan DS, Muecke J. Intravitreal aflibercept treatment in eyes with exudative age-related macular degeneration following prior treatment with intravitreal ranibizumab. Indian J Ophthalmol. 2015;63(11):832–6.
Cho H, Shah CP, Weber M, Heier JS. Aflibercept for exudative AMD with persistent fluid on ranibizumab and/or bevacizumab. Br J Ophthalmol. 2013;97(8):1032–5.
Thorell MR, Nunes RP, Chen GW, et al. Response to aflibercept after frequent re-treatment with bevacizumab or ranibizumab in eyes with neovascular AMD. Ophthalmic Surg Lasers Imaging Retina. 2014;45(6):526–33.
Gharbiya M, Iannetti L, Parisi F, De Vico U, Mungo ML, Marenco M. Visual and anatomical outcomes of intravitreal aflibercept for treatment-resistant neovascular age-related macular degeneration. Biomed Res Int. 2014;2014:273754.
Hall LB, Zebardast N, Huang JJ, Adelman RA. Aflibercept in the treatment of neovascular age-related macular degeneration in previously treated patients. J Ocul Pharmacol Ther. 2014;30(4):346–52.
Messenger WB, Campbell JP, Faridi A, et al. Injection frequency and anatomic outcomes 1 year following conversion to aflibercept in patients with neovascular age-related macular degeneration. Br J Ophthalmol. 2014;98(9):1205–7.
Pinheiro-Costa J, Costa JM, Beato JN, et al. Switch to aflibercept in the treatment of neovascular AMD: one-year results in clinical practice. Ophthalmologica. 2015;233(3–4):155–61.
Arcinue CA, Ma F, Barteselli G, Sharpsten L, Gomez ML, Freeman WR. One-year outcomes of aflibercept in recurrent or persistent neovascular age-related macular degeneration. Am J Ophthalmol. 2015;159(3):426–36.
De Massougnes S, Dirani A, Ambresin A, Delugis D, Marchionno L, Mantel I. Pigment epithelial detachment response to aflibercept in neovascular age-related macular degeneration refractory to ranibizumab. Retina. 2016;36:881–8.
Muftuoglu IK, Arcinue CA, Tsai FF, et al. Long-term results of pro re nata regimen of aflibercept treatment in persistent neovascular age-related macular degeneration. Am J Ophthalmol. 2016;167:1–9.
Ricci F, Parravano M, Regine F, et al. Aflibercept in persistent neovascular AMD: comparison of different treatment strategies in switching therapy. Eye (Lond). 2016;30(8):1077–83.
Van Lancker L, Petrarca R, Moutsouris K, Masaoutis P, Kampougeris G. Clinical experience of switching anti-VEGF therapy from ranibizumab to aflibercept in age-related choroidal neovascularization. Eur J Ophthalmol. 2017;27(3):342–5.
Waizel M, Todorova MG, Masyk M, et al. Switch to aflibercept or ranibizumab after initial treatment with bevacizumab in eyes with neovascular AMD. BMC Ophthalmol. 2017;17(1):79.
You Q, Gaber R, Meshi A, et al. High-dose high-frequency aflibercept for recalcitrant neovascular age-related macular degeneration. Retina. 2018;38:1156–65.
Dirani A, Mantel I. Ranibizumab treatment history as predictor of the switch-response to aflibercept: evidence for drug tolerance. Clin Ophthalmol. 2018;12:593–600.
Chatziralli I, Nicholson L, Vrizidou E, et al. Predictors of outcome in patients with neovascular age-related macular degeneration switched from ranibizumab to 8-weekly aflibercept. Ophthalmology. 2016;123(8):1762–70.
Kanesa-Thasan A, Grewal D, Gill M, Lyon A, Mirza R. Quantification of change in pigment epithelial detachment volume and morphology after transition to intravitreal aflibercept in eyes with recalcitrant neovascular AMD: 18-month results. Ophthalmic Surg Lasers Imaging Retina. 2015;46(6):638–41.
Barthelmes D, Campain A, Nguyen P, et al. Effects of switching from ranibizumab to aflibercept in eyes with exudative age-related macular degeneration. Br J Ophthalmol. 2016;100(12):1640–5.
Tyagi P, Juma Z, Hor YK, Scott NW, Ionean A, Santiago C. Clinical response of pigment epithelial detachment associated with neovascular age-related macular degeneration in switching treatment from ranibizumab to aflibercept. BMC Ophthalmol. 2018;18(1):148.
Homer N, Grewal DS, Mirza RG, Lyon AT, Gill MK. Transitioning to intravitreal aflibercept following a previous treat-and-extend dosing regimen in neovascular age-related macular degeneration: 24-month results. Eye (Lond). 2015;29(9):1152–5.
Ferrone PJ, Anwar F, Naysan J, et al. Early initial clinical experience with intravitreal aflibercept for wet age-related macular degeneration. Br J Ophthalmol. 2014;98(Suppl 1):i17–21.
Moon DRC, Lee DK, Kim SH, You YS, Kwon OW. Aflibercept treatment for neovascular age-related macular degeneration and polypoidal choroidal vasculopathy refractory to anti-vascular endothelial growth factor. Korean J Ophthalmol. 2015;29(4):226–32.
Cardoso P, Pinheiro AF, Meira J, et al. Switch to aflibercept in the treatment of neovascular AMD: long-term results. J Ophthalmol. 2017;2017:6835782.
Lee C, Kim A, Baughman D, et al. Visual acuity improvement when switching from ranibizumab to aflibercept is not sustained. Retina. 2017;1:1–6.
Amoaku WM, Chakravarthy U, Gale R, et al. Defining response to anti-VEGF therapies in neovascular AMD. Eye (Lond). 2015;29(6):721–31.
Abri Aghdam K, Seidensticker F, Pielen A, Framme C, Junker B. The short-term effects of aflibercept on the size of choroidal neovascularization lesion in treatment-resistant neovascular age-related macular degeneration as determined by spectral-domain optical coherence tomography. Lasers Surg Med. 2016;48(7):668–77.
Nomura Y, Yanagi Y. Intravitreal aflibercept for ranibizumab-resistant exudative age-related macular degeneration with choroidal vascular hyperpermeability. Jpn J Ophthalmol. 2015;59(4):261–5.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Theodoros Empeslidis: honoraria and travel grants from Novartis and Bayer. Theodoros Giannopoulos: research funding from Pharmaten and Bayer; travel support from Novartis and Bayer. Vassileios Konidaris: honoraria and travel grants from Novartis and Bayer. Paris G. Tranos: research funding from Novartis and Bayer; honoraria from Bayer, Allergan and Novartis. Irini C. Voudouragkaki: travel support from Vianex and Bayer. Anastasios G. Konstas: research funding from Allergan, Bayer and Santen; travel support from Vianex; honoraria from Allergan, Mundipharma, Santen and Vianex. Evangelia S. Panagiotou and Matthew Storey have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.8025965.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Empeslidis, T., Storey, M., Giannopoulos, T. et al. How Successful is Switching from Bevacizumab or Ranibizumab to Aflibercept in Age-Related Macular Degeneration? A Systematic Overview. Adv Ther 36, 1532–1548 (2019). https://doi.org/10.1007/s12325-019-00971-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-019-00971-0