Abstract
Introduction
We aimed to evaluate the safety and efficacy of loteprednol etabonate (LE) gel 0.5% compared with vehicle in the treatment of postoperative inflammation and pain following cataract surgery, using the integrated analysis of data from two identical, prospective, multicenter, randomized, double-masked, parallel-group, vehicle-controlled trials.
Methods
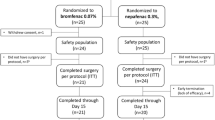
Patients with anterior chamber cell (ACC) inflammation ≥ grade 2 (6–15 cells) 1 day post-surgery were randomized to receive 1 or 2 drops of LE gel 0.5% or vehicle 4 times per day instilled in the study eye for 14 days. Primary outcome measures included the proportion of patients with complete resolution of ACC and grade 0 (no) pain on postoperative Day 8. Safety endpoints included adverse events (AEs), changes from baseline in intraocular pressure (IOP) and visual acuity (VA), biomicroscopy, and funduscopy findings. Gel comfort was graded by patients according to drop sensation.
Results
The intent-to-treat population included 813 patients (409 LE gel 0.5% and 404 vehicle). At postoperative Day 8, 30.8% and 15.1% of patients randomized to LE gel 0.5% or vehicle, respectively, had complete resolution of ACC, while 74.3% and 43.8% of patients, respectively, had grade 0 pain (P < 0.001 for both). Tolerability assessments for ocular itching, photophobia, and tearing favored LE gel 0.5% compared with vehicle at different time points beginning at Day 3. Two patients in the LE gel 0.5% group and 1 patient in the vehicle group exhibited a transient treatment-emergent increase in IOP ≥ 10 mmHg. Treatment-related AEs were generally mild to moderate and occurred less frequently with LE gel 0.5% than with vehicle. Reports of treatment-related blurred vision were rare (n = 2, vehicle).
Conclusion
LE gel 0.5% was efficacious and well tolerated in the treatment of postoperative pain and inflammation following ocular surgery, with minimal risk of IOP elevation.




Similar content being viewed by others
References
Ellwein LB, Urato CJ. Use of eye care and associated charges among the Medicare population: 1991–1998. Arch Ophthalmol. 2002;120:804–11.
Schein OD, Steinberg EP, Javitt JC, et al. Variation in cataract surgery practice and clinical outcomes. Ophthalmology. 1994;101:1142–52.
El-Harazi SM, Feldman RM. Control of intra-ocular inflammation associated with cataract surgery. Curr Opin Ophthalmol. 2001;12:4–8.
Mohammadpour M, Jafarinasab MR, Javadi MA. Outcomes of acute postoperative inflammation after cataract surgery. Eur J Ophthalmol. 2007;17:20–8.
Bartlett JD, Horwitz B, Laibovitz R, Howes JF. Intraocular pressure response to loteprednol etabonate in known steroid responders. J Ocul Pharmacol. 1993;9:157–65.
Clark AF. Basic sciences in clinical glaucoma: steroids, ocular hypertension, and glaucoma. J Glaucoma. 1995;4:354–69.
Chang DF, Tan JJ, Tripodis Y. Risk factors for steroid response among cataract patients. J Cataract Refract Surg. 2011;37:675–81.
Bartlett JD, Woolley TW, Adams CM. Identification of high intraocular pressure responders to topical ophthalmic corticosteroids. J Ocul Pharmacol. 1993;9:35–45.
Fingert JH, Clark AF, Craig JE, et al. Evaluation of the myocilin (MYOC) glaucoma gene in monkey and human steroid-induced ocular hypertension. Invest Ophthalmol Vis Sci. 2001;42:145–52.
Szabó V, Borgulya G, Filkorn T, Majnik J, Bányász I, Nagy ZZ. The variant N363S of glucocorticoid receptor in steroid-induced ocular hypertension in Hungarian patients treated with photorefractive keratectomy. Mol Vis. 2007;13:659–66.
The Loteprednol Etabonate Postoperative Inflammation Study Group 2. A double-masked, placebo-controlled evaluation of 0.5% loteprednol etabonate in the treatment of postoperative inflammation. Ophthalmology. 1998;105:1780–6.
Assil KK, Massry G, Lehmann R, Fox K, Stewart R. Control of ocular inflammation after cataract extraction with rimexolone 1% ophthalmic suspension. J Cataract Refract Surg. 1997;23:750–7.
Korenfeld MS, Silverstein SM, Cooke DL, et al. Difluprednate ophthalmic emulsion 0.05% for postoperative inflammation and pain. J Cataract Refract Surg. 2009;35:26–34.
Bodor N, Loftsson T, Wu WM. Metabolism, distribution, and transdermal permeation of a soft corticosteroid, loteprednol etabonate. Pharm Res. 1992;9:1275–8.
Bodor N, Buchwald P. Soft drug design: general principles and recent applications. Med Res Rev. 2000;20:58–101.
Wu WM, Huang F, Lee Y, Buchwald P, Bodor N. Pharmacokinetics of the sequential metabolites of loteprednol etabonate in rats. J Pharm Pharmacol. 2008;60:291–7.
Comstock TL, Decory HH. Advances in corticosteroid therapy for ocular inflammation: loteprednol etabonate. Int J Inflam. 2012;2012:789623.
Alberth M, Wu WM, Winwood D, Bodor N. LIpophilicity, solubility and permeability of loteprednol etabonate, a novel, soft anti-inflammatory steroid. J Biopharm Sci. 1991;2:115–25.
Druzgala P, Hochhaus G, Bodor N. Soft drugs–10. Blanching activity and receptor binding affinity of a new type of glucocorticoid: loteprednol etabonate. J Steroid Biochem Mol Biol. 1991;38:149–54.
Stahn C, Buttgereit F. Genomic and nongenomic effects of glucocorticoids. Nat Clin Pract Rheumatol. 2008;4:525–33.
Stewart R, Horwitz B, Howes J, Novack GD, Hart K. Double-masked, placebo-controlled evaluation of loteprednol etabonate 0.5% for postoperative inflammation. Loteprednol Etabonate Post-operative Inflammation Study Group 1. J Cataract Refract Surg. 1998;24:1480–9.
Comstock TL, Paterno MR, Singh A, Erb T, Davis E. Safety and efficacy of loteprednol etabonate ophthalmic ointment 0.5% for the treatment of inflammation and pain following cataract surgery. Clin Ophthalmol. 2011;5:177–86.
Fong R, Leitritz M, Siou-Mermet R, Erb T. Loteprednol etabonate gel 0.5% for postoperative pain and inflammation after cataract surgery: results of a multicenter trial. Clin Ophthalmol. 2012;6:1113–24.
Rajpal RK, Roel L, Siou-Mermet R, Erb T. Efficacy and safety of loteprednol etabonate 0.5% gel in the treatment of ocular inflammation and pain after cataract surgery. J Cataract Refract Surg. 2013;39:158–67.
Lotemax® Suspension 0.5% [Package insert]. Rochester, NY: Bausch and Lomb Inc; 2006.
Lotemax® Ointment 0.5% [Package Insert]. Rochester, NY: Bausch and Lomb Inc; 2011.
Bartlett JD, Howes JF, Ghormley NR, Amos JF, Laibovitz R, Horwitz B. Safety and efficacy of loteprednol etabonate for treatment of papillae in contact lens-associated giant papillary conjunctivitis. Curr Eye Res. 1993;12:313–21.
Asbell P, Howes J. A double-masked, placebo-controlled evaluation of the efficacy and safety of loteprednol etabonate in the treatment of giant papillary conjunctivitis. CLAO J. 1997;23:31–6.
Friedlaender MH, Howes J. A double-masked, placebo-controlled evaluation of the efficacy and safety of loteprednol etabonate in the treatment of giant papillary conjunctivitis. The Loteprednol Etabonate Giant Papillary Conjunctivitis Study Group I. Am J Ophthalmol. 1997;123:455–64.
Dell SJ, Shulman DG, Lowry GM, Howes J. A controlled evaluation of the efficacy and safety of loteprednol etabonate in the prophylactic treatment of seasonal allergic conjunctivitis. Loteprednol Allergic Conjunctivitis Study Group. Am J Ophthalmol. 1997;123:791–7.
Shulman DG, Lothringer LL, Rubin JM, et al. A randomized, double-masked, placebo-controlled parallel study of loteprednol etabonate 0.2% in patients with seasonal allergic conjunctivitis. Ophthalmology. 1999;106:362–9.
Amon M, Busin M. Loteprednol etabonate opththalmic suspension 0.5%: efficacy and safety for postoperative anti-inflammatory use. Int Ophthalmol. 2012;32:507–17.
Walters TR, Goldberg DF, Peace JH, Gow JA. Bromfenac ophthalmic solution 0.07% (Prolensa) dosed once daily for cataract surgery: results of 2 randomized controlled trials. Ophthalmology. 2013 [Epub ahead of print].
Silverstein SM, Cable MG, Sadri E, et al. Once daily dosing of bromfenac ophthalmic solution 0.09% for postoperative ocular inflammation and pain. Curr Med Res Opin. 2011;27:1693–703.
Donnefeld ED, Nichamin LD, Hardten DR, et al. Twice-daily, preservative-free ketorolac 0.45% for treatment of inflammation and pain after cataract surgery. Am J Ophthalmol. 2011;151:420–6.
McGhee CN, Dean S, Danesh-Meyer H. Locally administered ocular corticosteroids: benefits and risks. Drug Saf. 2002;25:33–55.
Holland EJ, Bartlett JD, Paterno MR, et al. Effects of loteprednol/tobramycin versus dexamethasone/tobramycin on intraocular pressure in healthy volunteers. Cornea. 2008;27:50–5.
Rajpal RK, Digby D, D’Aversa G, Mah F, Hollander DA, Conway T. Intraocular pressure elevations with loteprednol etabonate: a retrospective chart review. J Ocul Pharmacol Ther. 2011;27:305–8.
Khoh-Reiter S, Jessen BA. Evaluation of the cytotoxic effects of ophthalmic solutions containing benzalkonium chloride on corneal epithelium using an organotypic 3-D model. BMC Ophthalmol. 2009;9:5.
Noecker RJ, Herrygers LA, Anwaruddin R. Corneal and conjunctival changes caused by commonly used glaucoma medications. Cornea. 2004;23:490–6.
Pellinen P, Huhtala A, Tolonen A, Lokkila J, Mäenpää J, Uusitalo H. The cytotoxic effects of preserved and preservative-free prostaglandin analogs on human corneal and conjunctival epithelium in vitro and the distribution of benzalkonium chloride homologs in ocular surface tissues in vivo. Curr Eye Res. 2012;37:145–54.
Comstock TL, Usner DW. Effect of loteprednol etabonate ophthalmic suspension 0.5% on post-operative pain and discomfort. In: Presented at the 2010 ASCRS Symposium and Congress on Cataract, IOL, and Refractive Surgery; April 9–14, 2010. Boston, MA.
Acknowledgments
Bausch and Lomb, Inc. sponsored the two original studies in this pooled analysis and were responsible for the design and conduct of the study as well as data collection, management, and analysis of study data, and together with lead investigators, interpretation of the data, and preparation, review and approval of the manuscript. Bausch and Lomb, Inc. also funded the article publication charges for this study. The authors wish to thank Carol Tozzi, PhD, of Churchill Communications (Maplewood, NJ) for writing assistance (developing a first draft). This assistance was funded by Bausch and Lomb, Inc. The authors retained full control of the manuscript content.
Conflict of interest
T. Comstock is an employee of Bausch and Lomb, Inc. R. Rajpal is a consultant and speaker for Bausch and Lomb and has received grant support, honorarium and support for travel to meetings.
Compliance with ethics guidelines
All patients were required to provide written informed consent. Ethical approval was obtained from Schulman Associates IRB, Incorporated (Cincinnati, Ohio). Both studies were conducted in accordance with the World Medical Association Declaration of Helsinki (revised edition, 2004), relevant ICH Harmonized Tripartite Guidelines for Good Clinical Practice 1996, HIPAA guidelines, and applicable local regulations. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 and 2008.
Author information
Authors and Affiliations
Corresponding author
Additional information
Clinicaltrials.gov NCT 01010633 and NCT 01060072.
Rights and permissions
About this article
Cite this article
Rajpal, R.K., Fong, R. & Comstock, T.L. Loteprednol Etabonate Ophthalmic Gel 0.5% Following Cataract Surgery: Integrated Analysis of Two Clinical Studies. Adv Ther 30, 907–923 (2013). https://doi.org/10.1007/s12325-013-0059-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-013-0059-7