Abstract
Introduction
Coronary computed-tomography angiography (CCTA) has high diagnostic performance, but it sometimes does not allow evaluation because of artifacts. Currently, the use of a β-blocker is recommended to prevent motion artifacts, but the β-blocker (metoprolol, propranolol, etc.) commonly used has a slow onset and long duration of action. Landiolol hydrochloride is an intravenous β1-blocker with a very short half-life. We investigated the efficacy and optimal dose of this drug for reduction of heart rate in patients undergoing CCTA.
Methods
Eighty-seven subjects with ischemic heart disease were divided into three groups to receive landiolol hydrochloride at a dose of 0.125 (Group L), 0.25 (Group M), or 0.5 mg/kg (Group H). CCTA was performed at 3–7 min after administration, and heart rate, blood pressure, and image quality were assessed.
Results
Heart rate decreased rapidly after completion of landiolol hydrochloride administration in all groups, with a heart rate reduction of 15.55 ± 6.56% in Group L, 16.48 ± 7.80% in Group M, and 21.49 ± 6.13% in Group H (Group L vs Group H, P = 0.0008; Group M vs Group H, P = 0.0109). Since there was no significant difference in heart rate during imaging among the three groups, although there was a significant difference between groups L and H and groups M and H in terms of percent change in heart rate, coronary stenosis was diagnosable in all groups with no significant difference.
Conclusion
Landiolol hydrochloride showed a rapid onset and short β-blocking effect, and was most effective at a dose of 0.5 mg/kg. However, the diagnosable proportion had no significant differences among the three groups in CCTA. Therefore, the clinically recommended dose was 0.125 mg/kg or less, considering the heart rate of patients with suspected coronary stenosis during CCTA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronary angiography (CAG) has been used for the diagnosis of coronary stenosis or occlusion, which causes ischemic heart disease, including angina pectoris and acute myocardial infarction. With the development of medical devices, marked progress has been made recently in non-invasive diagnostic imaging. In particular, coronary computed-tomography angiography (CCTA), using multidetector row computed tomography (MDCT), has proved to be an excellent imaging modality for the diagnosis of cardiovascular disease as it not only has high spatial resolution, but also allows imaging with only one breath hold [1–3]. Using a non-invasive approach, CCTA provides morphological information equivalent to that obtained by CAG, and also has very high diagnostic performance for cardiovascular disease. However, although CCTA has high diagnostic performance in clinical settings, it does not allow assessment in some patients because of artifacts associated with calcification (beam-hardening effect) or the influence of heart beat (motion artifact) [4–6]. Solutions to these artifacts have been sought and attempts have been made to eliminate artifacts due to calcification using dual energy [7, 8] or subtraction [9–11]. As well as eliminating motion artifacts, sufficient time resolution is required to visualize the beating heart in a resting state. Time resolution of computed tomography (CT) equipment depends on the rotation speed of the X-ray tube and detector. Since the rotation speed is eventually limited, images are likely to be affected by heart beats in patients with a high heart rate [12]. In order to reduce the time resolution by half, dual source CT equipment with two tubes and two detectors has been developed [13]. However, it has not proved popular, because its use is limited to heart imaging only. Current CCTA guidelines published by the Society of Cardiovascular Computed Tomography recommend the use of β-blockers to prevent motion artifacts due to heart beats [14–19]. However, metoprolol, a commonly prescribed highly β1-selective drug, achieves its maximum β-blocking effect at 1–2 h after administration, with a duration of at least 6–8 h [20, 21].
Landiolol hydrochloride, (−)-[(S)-2,2-dimethyl-1,3-dioxolan-4-yl] methyl 3-{4-[(S)-2-hydroxy-3-(2-morpholinocarbonylamino)ethylamino] propoxy} phenylpropionate monohydrochloride, is a β1-blocker developed by Ono Pharmaceutical Co., Ltd. Osaka, Japan, in 1991. Having an ester bond, it has a short plasma half-life, during which it is metabolized and inactivated by esterases in the blood and liver. Furthermore, landiolol hydrochloride has high selectivity for β1 receptors, with a β1/β2 ratio of approximately 250 [22, 23].
The optimal dose of β-blockers varies according to race [24, 25]. Since landiolol hydrochloride has a rapid onset and short β-blocking effect, we considered that this drug would be effective for controlling heart rate during CCTA. On this basis, we determined the optimal dose of landiolol hydrochloride in Japanese subjects with suspected ischemic heart disease undergoing CCTA in an open-label comparative study.
Methods
Study Population
Prior to CCTA, 90 Japanese patients aged ≥20 years with suspected ischemic cardiac disease were selected based on symptoms recorded by a physician, physical examination, standard 12-lead electrocardiogram (ECG), chest X-ray, and echocardiography findings. The patients were eligible for inclusion in the study if they presented with a stable angina syndrome, had been referred for clinically indicated CCTA, and had a heart rate of 70–90 beats/min on admission to the CT room and immediately before administration of a nitrate vasodilator drug.
Patients were excluded from the study if they had a cardiac pacemaker and/or defibrillator; had undergone coronary-artery bypass surgery; had atrial fibrillation or extrasystoles at imaging; or were pregnant, lactating, or possibly pregnant or desiring to become pregnant during the study period. Furthermore, the use of β receptor blockers, non-ionic contrast media, dihydropyridine or non-dihydropyridine calcium antagonists (diltiazem hydrochloride, verapamil hydrochloride, bepridil hydrochloride), antiarrhythmic agents, sympathomimetic agents and biguanide antidiabetic drugs was contraindicated during the study.
The appropriateness of the study was reviewed and approved by the Institutional Review Board at each study center, before initiating the study. The study was conducted in accordance with the ethical principles in the Declaration of Helsinki, and in compliance with the Pharmaceutical Affairs Law and the Ordinance on Standards for Implementation of Clinical Studies on Drugs (Ministry of Health and Welfare Ordinance No. 28) in Japan. Prior to participation in the study all patients provided written informed consent. The study was conducted between April and December 2006.
Study Design
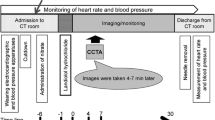
This was an open-label, multicenter study, conducted at seven centers in Japan. The study methodology is described at http://www.clinicaltrials.gov/ct2/results?term=NCT00311038&Search=Search. Prior to CCTA, eligible subjects were enrolled into one of three landiolol hydrochloride treatment groups: 0.125 mg/kg (low-dose, n = 30), 0.25 mg/kg (medium-dose, n = 30) and 0.5 mg/kg (high-dose, n = 30). Administration of landiolol hydrochloride was started in the low-dose cohort, and then subsequently carried out in the medium- and high-dose cohorts, respectively. Landiolol hydrochloride was administered as an intravenous (IV) bolus injection over 1 min after administration of nitroglycerin (0.3–0.6 mg) under the tongue. This was then followed by CCTA imaging/monitoring 3–7 min later (Fig. 1).
Dose Selection
In a Phase I study in healthy volunteers, a landiolol hydrochloride dose of 0.5 mg/kg was found to be the maximum dose that did not cause any abnormalities or variations in signs/symptoms, ECG findings, physiological tests, or laboratory tests, and was well tolerated [26]. On this basis, a landiolol hydrochloride dose of 0.5 mg/kg was selected for the high-dose group in the current study.
In a phase III study [27] in patients with atrial fibrillation/flutter, the optimal dose of landiolol hydrochloride in terms of efficacy and safety was 0.25 mg/kg. For the low-dose group, a landiolol hydrochloride dose that was equivalent to half the optimal dose was selected (i.e. 0.125 mg/kg). Accordingly, three dose levels of 0.125 mg/kg (Group L), 0.25 mg/kg (Group M), and 0.5 mg/kg (Group H) were selected for the present study to find the optimal dose of landiolol hydrochloride for patients undergoing CCTA.
CCTA
CCTA was conducted at 3–7 min after completion of administration of the study drug, to assess the improvement in coronary visualization achieved after administration of landiolol hydrochloride (Fig. 1). The SOMATOM Sensation Cardiac 64 CT system (Siemens Japan CO., LTD., Tokyo, Japan) was used and operated at the following settings: X-ray tube voltage 120 kV, tube current 770–850 mA s, collimation 32 rows × 0.6 mm, X-ray tube rotation speed 0.33 s/rot, helical pitch 0.2, and field of view 200 mm. A non-ionic contrast medium, iopamidol (370 mg I/mL), was administered IV at 3–4.5 mL/s using a 2-channel injector, followed by an IV infusion of saline (20–30 mL) using a bolus tracking system.
Image reconstruction was performed according to the retrospective ECG-gated reconstruction method [28], under optimal conditions at each study center, using a slice thickness for reconstruction of 0.75 mm.
Image Analysis
At the core laboratory, volume-rendering images, curved multi-planar reformation (MPR) images, interactive oblique MPR images, thin maximum intensity projection (MIP) images and cross-sectional images were prepared using the images reconstructed at each study center. All images of the 15 coronary segments, based on the American Heart Association classification [29], were assessed and classified by a central coronary visualization judgment committee which consisted of three independent radiodiagnostic specialists. The images were given a quality score of either 1 (motion artifact(s) present and impossible to diagnose); 2 (motion artifact(s) present but diagnosable); or 3 (no motion artifact and diagnosable).
Preparation of the images as well as assessment of the diagnosable proportion was performed using a workstation, Aquarius NET Server (TeraRecon, Inc. California, USA).
Endpoints
The primary efficacy endpoint was the diagnosable proportion (proportion of subjects whose coronary stenosis was diagnosable in reconstructed images).
Additional endpoints investigated included: the extent and duration of effect of landiolol hydrochloride on heart rate [mean, the achieve proportion (proportion of subjects who achieved the threshold of 60, 65 or 70 beats/min), and percent change] and blood pressure, percutaneous oxygen saturation (SpO2), ECG parameters, and adverse events. Heart rate (Holter ECG), blood pressure and SpO2 were monitored before study initiation (baseline: measured on the day of CCTA), on admission to the CT room, immediately before administration of nitroglycerin, immediately before administration of the study drug, and at 1-min intervals from 0 to 10 min, and at 15 and 30 min, after completion of study drug administration, In addition, a 12-lead ECG was performed and laboratory values were measured before study initiation (baseline) and within 3 days after completion of study drug administration. And, we investigated the relationship between the diagnosable proportion and heart rate in order to confirm the image quality-improving effect of administration of landiolol hydrochloride.
Adverse events (AEs) were recorded from the start of study drug administration until the end of the monitoring period (within 3 days after completion of study drug administration).
Statistical Analysis
The study population was selected based on the assumption that the heart rate reduction was 10.0 ± 12.0%, using paired t test (significance level: 0.05, two-tailed) with a power of 80%, so that subjects with a significant difference could be detected. Analysis of the primary efficacy endpoint, was based on the per protocol analysis set (PPS) and the safety analysis was based on the full analysis set (FAS). PPS is restricted to the participants who fulfill the protocol in the terms of the eligibility, interventions, and outcome assessment. FAS was restricted to the participants who fulfill the main protocol in the terms of the eligibility, interventions, and outcome assessment. Intergroup differences in patient baseline characteristics were assessed using the Kruskal–Wallis or Chi-squared test. Intergroup differences in efficacy endpoints were assessed using the Chi-squared test, Cochran–Armitage trend test, t test, contrast t test, Wilcoxon rank sum test, and Cochran–Mantel–Haenszel test. Intergroup differences in safety endpoints were assessed using the Kruskal–Wallis test or Chi-squared test. The level of significance was set at 5% (two-tailed) or 2.5% (one-tailed).
Results
Subject Disposition and Baseline Characteristics
All 90 subjects enrolled in the study received the study drug, without any withdrawals or dropouts. However, two subjects did not meet the inclusion criteria, and one subject had incomplete data for assessment. As a result, 87 subjects were included in the PPS analysis set (low-dose, n = 29; medium-dose, n = 28; high-dose, n = 30) (Fig. 2). There was no significant difference among the three groups for patient baseline characteristics (Table 1).
Heart Rate During CCTA
The data of heart rate during CCTA is shown in Table 2. Mean heart rate [±standard deviation (SD)] during imaging was 63.9 ± 7.8, 64.0 ± 8.1, and 62.8 ± 9.4 beats/min in Group L, Group M and Group H, respectively. The percentage of subjects whose heart rate decreased below 60 beats/min during imaging was 24.1% (n = 7) in Group L, 28.6% (n = 8) in Group M and 43.3% (n = 13) in Group H. The corresponding percentage of subjects with a reduction in heart rate to below 65 beats/min during imaging was 58.6% (n = 17), 60.7% (n = 17), and 63.3% (n = 19), respectively, and with a reduction to below 70 beats/min during imaging was 75.9% (n = 22), 78.6% (n = 22) and 73.3% (n = 22), respectively.
Although the threshold (heart rate <60 or 65 beats/min) was reached in a higher percentage of subjects in Group H than in Group L or Group M, there was no significant difference in the percentage of subjects achieving the individual threshold among the three groups.
Percent Change in Heart Rate After Administration of Landiolol Hydrochloride
Following administration of landiolol hydrochloride, there was a dose-dependent reduction in heart rate which was highest in Group H (15.55 ± 6.56% in Group L, 16.48 ± 7.80% in Group M, and 21.49 ± 6.13% in Group H). The difference between dose groups reached statistical significance between Group L and Group H and between Group M and Group H (Table 2).
Heart Rate Evaluation
Heart rate began to decrease immediately after completion of study drug administration in all three dose groups (Fig. 3). The lowest heart rate was achieved within 3 min after completion of administration in 41.3% of subjects in Group L, 39.3% in Group M and 53.3% in Group H, within 5 min in 68.9% of subjects in Group L, 75.0% in Group M and 83.3% in Group H, and within 7 min in 72.3% of the subjects in Group L, 82.1% in Group M and 89.9% in Group H. The time (mean ± SD) required to reach the lowest heart rate was 5.0 ± 2.8 min in Group L, 4.5 ± 2.5 min in Group M and 3.4 ± 2.1 min in Group H. The time decreased with an increase in dose, and heart rate returned to baseline levels at 30 min after completion of landiolol hydrochloride administration.
Blood Pressure Evaluation
In all three dose groups, both systolic and diastolic blood pressure showed the greatest decrease at 3–4 min after completion of landiolol hydrochloride administration, although the percent decrease was minimal (Fig. 4). Systolic and diastolic blood pressure returned to baseline levels in all groups at 30 min after completion of administration.
Image Quality
The percentage of subjects with an image quality score of ≥2 was 61.5% (n = 16) in Group L, 44.4% (n = 12) in Group M and 55.2% (n = 16) in Group H. There was no significant difference between the three dose groups, indicating that image quality was sufficiently improved in the low-dose group (Table 3).
Analysis of image quality by coronary vessel revealed a significant difference between Group L and Group M (Wilcoxon rank sum test, P = 0.0461), whereas analysis by coronary segment revealed a significant difference between Group L and Group M (P < 0.0001) and between Group L and Group H (P = 0.0007). Since there was no significant difference in heart rate during imaging among the three groups, although there was a significant difference between groups L and H and groups M and H in terms of percent change in heart rate, the subjects were classified by heart rate to determine the percentage of subjects with an image quality score of ≥2. Using this approach, a definite correlation was noted between reduction in heart rate and image quality score (≥2) (Fig. 5).
Safety
There was no dose-dependent increase in the incidence of AEs, adverse drug reactions or decreased blood pressure, showing no significant difference among the three groups (Chi-squared test, P = 0.7698). Six AEs were reported in five subjects: a mild increase in alanine aminotransferase (1 patient in low-dose group), a mild increase in aspartate aminotransferase (1 patient in low-dose group), a mild reduction of blood platelets (1 patient in low-dose group), a moderate increase in blood creatine phosphokinase (1 patient in middle-dose group), a mild reduction in blood pressure (1 patient in middle-dose group), and a mild increase in γ-glutamic acid transferase (1 patient in high-dose group). All of the AEs, excluding the moderate increase in blood creatine phosphokinase, were considered possibly related to the landiolol hydrochloride, but resolved without any treatment, with no abnormalities in SpO2 or ECG parameters.
Reduction in blood pressure was reported as an AE in one subject (3.3%) in Group M and in none of the subjects in Group L or Group H, (P = 0.3638 between groups). The subject with a reduction in blood pressure in Group M experienced a mild decrease which began immediately after completion of study drug administration and was lowest 7 min after completion of administration, indicating a causal relationship with landiolol hydrochloride. However, this subject did not experience any symptoms associated with decreased blood pressure, and the effect resolved without any treatment 15 min after onset.
No abnormalities were noted in any ECG parameters (RR interval, PQ interval, QRS width, QT interval, ST segment).
Discussion
CCTA has high diagnostic performance in diagnosable cases, but does not allow assessment in some patients because of artifacts due to calcification or heart beats [4–6]. Imaging of the beating heart requires sufficient time resolution to allow imaging of the heart in a resting state. However, many models of CT equipment do not have sufficient time resolution for imaging without being affected by motion artifacts. Currently, the most popular model used for CCTA is 64-MDCT, but this model does not have sufficient time resolution to eliminate the influence of heart beats. Many studies have recommended a heart rate of 65 beats/min or less to eliminate the effect of motion artifact [17, 18, 32]. The mean resting heart rate was reported to be 74 beats/min in 28,507 adults in the general Norwegian population, and 76.3 beats/min in 16,955 patients with hypertension in United States [30, 31]. Therefore, in order to improve relative time resolution, it is necessary to reduce heart rate using β-blockers [14–16].
In other words, during CCTA imaging, a β-blocker that is able to reduce the heart rate from approximately 75 beats/min to less than 65 beats/min is necessary. The present study showed that the heart rate-lowering effect of landiolol hydrochloride was dose dependent, but the clinically recommended dose was concluded to be 0.125 mg/kg or less, considering the heart rate of patients with suspected coronary stenosis during CCTA.
Landiolol hydrochloride is a β1-blocker characterized by a very short half-life of approximately 4 min [26]. Since traditional β-blockers have long half-lives and a prolonged effect after CCTA imaging, they have been associated with safety problems, such as a reduction of blood pressure. However, landiolol hydrochloride showed a rapid onset of heart rate lowering after administration, with rapid recovery of heart rate after completion of the examination. Since this drug decreased heart rate only during CCTA, owing to its rapid onset and short duration of β-blocking effect, it seems to be a suitable β-blocking agent for use prior to CCTA. The diagnosable proportion had no significant difference between the three dose groups in CCTA for assessment of the severity of coronary stenosis.
Beta-blockers with a heart rate-lowering effect were originally used as antihypertensive agents, suggesting that a decrease in blood pressure is inevitable with the use of β-blockers. In this respect, landiolol hydrochloride differs markedly from conventional β-blockers in that it produces only a very small decrease in blood pressure [22, 23]. In the present study, landiolol hydrochloride did not cause a large decrease in blood pressure during CCTA imaging. Furthermore, owing to its very short half-life, the effect of this drug was rapidly eliminated even if a subject experienced a transient decrease of blood pressure, indicating the safety of this drug. Actually, in the present study, the patients with a mild decrease of blood pressure in a middle-dose group has recovered soon without any treatment. In the safety evaluation, landiolol hydrochloride was not repeated to cause any serious adverse events. Therefore, landiolol hydrochloride is considered to be well tolerated at doses between 0.125 and 0.5 mg/kg.
There was no significant difference in the proportion of subjects with image quality scores of 2 or more among the three groups. Image quality has been reported to correlate with a reduction in heart rate to some extent [1–4]. After administration of landiolol hydrochloride during the present study, although there was a significant difference between groups L and H and groups M and H in terms of percent change in heart rate, no significant difference was noted in the heart rate during imaging among the three groups. Therefore, it was a natural outcome that no significant difference in image quality was detected among the three groups. The limitations of the present study was that we did not directly compare the safety and efficacy between this drug and conventional beta-blockers (oral or intravenous) or placebo, which is expected to be evaluated in further studies.
Conclusion
Landiolol hydrochloride showed a rapid onset and short duration of heart rate lowering, and was found to be most effective at a dose of 0.5 mg/kg. However, the diagnosable proportion did not show significant differences among the three groups in CCTA. Therefore, the clinically recommended dose was 0.125 mg/kg or less, considering the heart rate of patients with suspected coronary stenosis during CCTA.
References
Raff GL, Gallagher MJ, O’Neill WW, Goldstein JA. Diagnostic accuracy of noninvasive coronary angiography using 64-slice spiral computed tomography. J Am Coll Cardiol. 2005;46:552–7.
Garcia MJ, Lessick J, Hoffmann MH, CATSCAN Study Investigators. Accuracy of 16-row multidetector computed tomography for the assessment of coronary artery stenosis. JAMA. 2006;296:403–11.
Mollet NR, Cademartiri F, van Mieghem CA, et al. High-resolution spiral computed tomography coronary angiography in patients referred for diagnostic conventional coronary angiography. Circulation. 2005;112:2318–23.
Miller JM, Rochitte CE, Dewey M, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008;359:2324–36.
Meijboom WB, Meijs MF, Schuijf JD, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008;52:2135–44.
Mowatt G, Cook JA, Hillis GS, et al. 64-Slice computed tomography angiography in the diagnosis and assessment of coronary artery disease: systematic review and meta-analysis. Heart. 2008;94:1386–93.
Ruzsics B, Lee H, Zwerner PL, Gebregziabher M, Costello P, Schoepf UJ. Dual-energy CT of the heart for diagnosing coronary artery stenosis and myocardial ischemia—initial experience. Eur Radiol. 2008;18:2414–24.
Kaza RK, Platt JF, Cohan RH, Caoili EM, AI-Hawary MM, Wasnik A. Dual-energy CT with single- and dual-source scanners: current applications in evaluating the genitourinary tract. Radiographics. 2012;32:353–69.
Takahashi N, Hartman RP, Vrtiska TJ, et al. Dual-energy CT iodine-subtraction virtual unenhanced technique to detect urinary stones in an iodine-filled collecting system: a phantom study. AJR Am J Roentgenol. 2008;190:1169–73.
Badea CT, Drangova M, Holdsworth DW, Johnson GA. In vivo small animal imaging using micro-CT and digital subtraction angiography. Phys Med Biol. 2008;53:R319–50.
Yoshioka K, Tanaka R, Muranaka K. Subtraction coronary CT angiography for calcified lesions. Cardiol Clin. 2012;30:93–102.
Giesler T, Baum U, Ropers D, Ulzheimer S, Wenkel E, Mennicke M, et al. Noninvasive visualization of coronary arteries using contrast-enhanced multidetector CT: influence of heart rate on image quality and stenosis detection. Am J Roentgenol. 2002;179:911–6.
Ropers U, Ropers D, Pflederer T, et al. Influence of heart rate on the diagnostic accuracy of dual-source computed tomography coronary angiography. J Am Coll Cardiol. 2007;50:2393–8.
Jacobs JE, Boxt LM, Desjardins B, Fishman EK, Larson PA, Schoepf J, American College of Radiology. ACR practice guideline for the performance and interpretation of cardiac computed tomography (CT). J Am Coll Radiol. 2006;3:677–85.
Budoff MJ, Achenbach S, Blumenthal RS, et al. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006;114:1761–91.
Budoff MJ, Dowe D, Jollis JG, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52:1724–32.
Bluemke DA, Achenbach S, Budoff M, et al. Noninvasive coronary artery imaging: magnetic resonance angiography and multidetector computed tomography angiography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention, and the Councils on Clinical Cardiology and Cardiovascular Disease in the Young. Circulation. 2008;118:586–606.
American College of Cardiography Foundation Task Force on Expert Consensus Documents, Mark DB, Berman DS, Budoff MJ, et al. ACCF/ACR/AHA/NASCI/SAIP/SCAI/SCCT 2010 expert consensus document on coronary computed tomographic angiography: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation 2010;121:2509–43.
Abbara S, Arbab-Zadeh A, Callister TQ, et al. SCCT guidelines for performance of coronary computed tomographic angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2009;3:190–204.
Sakakibara H, Miyatake K, Asao M, Ohara T. Effect of metoprolol on blood pressure and heart rate at rest and during exercise in healthy volunteer—the consideration by double-blind, cross-over method—Yakuri to Chiryou. 1979;7:1334–40. (in Japanese).
Regardh CG, Johnsson G, Jordö L, Sölvell L. Comparative bioavailability and effect studies on metoprolol administered as ordinary and slow-release tablets in single and multiple doses. Acta Pharmacol Toxicol. 1975;36:45–58.
He Q, Shi M, Liu X, et al. Determination of landiolol, an ultra-short-acting β1-receptor antagonist, in human plasma by liquid chromatography–tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;891–892:7–11.
Iguchi S, Iwamura H, Nishizaki M, et al. Development of a highly cardioselective ultra short-acting β-blocker, ONO-1101. Chem Pharm Bull (Tokyo). 1992;40:1462–9.
Kalow W. Pharmacogenetics in biological perspective. Pharmacol Rev. 1997;49:369–79.
Mahgoub A, Idle JR, Dring LG, Lancaster R, Smith RL. Polymorphic hydroxylation of debrisoquine in man. Lancet. 1977;17:584–6.
Nakashima M, Kanemaru M. Phase I study of ONO-1101, a new ultra short acting β1-blocking agent in healthy volunteers. J Clin Ther Med 2000;16:1531–56. (in Japanese).
Kato K, Hayakawa H, Atarashi H, et al. Clinical effect of intravenous infusion of landiolol hydrochloride (ONO-1101) on paroxysmal atrial fibrillation and atrial flutter—a phase III, double-blind study in comparison with placebo. J Clin Ther Med 1997;13:4903–24. (in Japanese).
Achenbach S, Ulzheimer S, Baum U, Kachelriess M, Ropers D, Giesler T, et al. Noninvasive coronary angiography by retrospectively ECG-gated multislice spiral CT. Circulation. 2000;102:2823–8.
Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975;51:5–40.
Nauman J, Janszky I, Vatten LJ, Wisløff U. Temporal changes in resting heart rate and deaths from ischemic heart disease. JAMA. 2011;306:2579–87.
Farinaro E, Stranges S, Guglielmucci G, et al. Heart rate as a risk factor in hypertensive individuals. The Italian TensioPulse Study. Nutr Metab Cardiovasc Dis. 1999;9:196–202.
Lesser JR, Flygenring B, Knickelbine T, et al. Clinical utility of coronary CT angiography: coronary stenosis detection and prognosis in ambulatory patients. Catheter Cardiovasc Interv. 2007;69:64–72.
Acknowledgments
The authors thank all the investigators (listed below) and site staff who participated in this clinical trial. Dr. Masaharu Hirano is the guarantor for this article, and takes responsibility for the integrity of the work as a whole. Sponsorship and article processing charges for this study was funded by Ono Pharmaceutical Co. Ltd.
Conflict of interest
Masaharu Hirano received consulting fees from Ono Pharmaceutical Co., Ltd. Kazuhiro Hara received consulting fees from Ono Pharmaceutical Co., Ltd. Yuji Ikari received consulting fees from Ono Pharmaceutical Co., Ltd. Masahiro Jinzaki received consulting fees from Ono Pharmaceutical Co., Ltd. Misako Iino received consulting fees from Ono Pharmaceutical Co., Ltd. Chikuma Hamada received consulting fees from Ono Pharmaceutical Co., Ltd. Sachio Kuribayashi received consulting fees from Ono Pharmaceutical Co., Ltd.
Compliance with ethics guidelines
The appropriateness of the study was reviewed and approved by the Institutional Review Board at each study center, before initiating the study. The study was conducted in accordance with the ethical principles in the Declaration of Helsinki, and in compliance with the Pharmaceutical Affairs Law and the Ordinance on Standards for Implementation of Clinical Studies on Drugs (Ministry of Health and Welfare Ordinance No. 28) in Japan. Prior to participation in the study, all patients provided written informed consent.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Appendices
Principal Investigators
Mitsuharu hatori, Daisuke Abe, Yawara Niijima, Toshiaki Ando, Yushin Muranaka, Akira Yamashina, Masaharu Hirano, Masao Yamada, Kazuhiro Hara, Yoshinobu Onuma, Hirosada Yamamoto, Atsuhiko Yagishita, Sen Yachi, Shuji Ohtsuki, Ruri Chihara, Kengo Tanabe, Shigeru Saito, Yusuke Miyashita, Takaaki Shiono, Hideaki Kaneda, Saeko Takahishi, Hideyuki Koga, Junya Matsumi, Hirosi Dohmae, Shingo Mizuno, Kazuya Sugitatsu, Yoshio Taketani, Yoshiyasu Minami, Shinji Tanaka, Hiroshi Inoue, Tomoki Kameyama, Michihisa Jogasaki, Shinichi Minagoe, Tatsuru Matsuoka, Hitoshi Nakashima, Manabu Setoguchi, Masahiro Sonoda, Hideki Tanaka.
ONO Pharmaceutical Clinical Development Team
Mitsunobu Tanimoto, Tatsuaki Okamura, Hiroshi Inose, Tomohiro Katsuno, Morito Akisada, Akira Tsuchiya (data manager), Masahiro Yoshizaki (statistician), and Shinichi Kikawa.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Hirano, M., Hara, K., Ikari, Y. et al. Dose-Finding Study of Landiolol Hydrochloride: A Short-Acting β1-Blocker for Controlling Heart Rate During Coronary Computed-Tomography Angiography in Japan. Adv Ther 30, 803–818 (2013). https://doi.org/10.1007/s12325-013-0053-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-013-0053-0