Abstract
Introduction
Streptococcus pneumoniae is the leading cause of bacterial meningitis. Young children, the elderly and those who are immunocompromised or who suffer from chronic diseases have the highest risk of developing pneumococcal meningitis. A 7-valent pneumococcal conjugate vaccine (PCV7) was licensed in 2000 in the US and in 2001 in Europe.
Methods
A literature search was performed in PubMed to identify studies assessing the impact of routine childhood PCV7 vaccination on pneumococcal diseases. Here, we report the impact on pneumococcal meningitis.
Results
A total of 17 articles reporting impact data on pneumococcal meningitis were included in this review: 11 from Western Europe and 6 from North America. In the post-vaccination period, compared with the pre-vaccination period, a reduction ranging from 59.2% in the US, 1 year after vaccine introduction, to 100% in Belgium, 4 years after vaccine introduction in vaccine-type (VT) pneumococcal meningitis incidence was reported in vaccine-eligible children in seven studies. In addition, the majority of studies reported reductions in VT and all-type pneumococcal meningitis incidence in age groups that were not vaccine-eligible.
Conclusions
The results from this review demonstrate that PCV7 has had a significant impact on pneumococcal meningitis across all ages through its use in pediatric immunization programs. With the introduction of 13-valent PCV (PCV13) we can expect to see a reduction in the incidence of pneumococcal meningitis due to the six additional serotypes included, as well as continued protection against pneumococcal meningitis due to PCV7 serotypes. Robust surveillance systems are essential for the evaluation of the impact of PCV13 on all-type pneumococcal meningitis and for monitoring the evolution of non-vaccine serotype pneumococcal meningitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Streptococcus pneumoniae is the leading cause of bacterial meningitis [1]. The risk of developing pneumococcal meningitis is highest in young children, the elderly and those who are immunocompromised or who suffer from chronic diseases [1]. In a global literature review of children under 5 years of age, the overall incidence rate of pneumococcal meningitis was 17/100,000 (95% CI 8–21), ranging from 6/100,000 (95% CI 5–9) in Europe to 38/100,000 (95% CI 11–48) in Africa [2]. In Europe, before the widespread use of 7-valent pneumococcal conjugate vaccine (PCV7), the highest pneumococcal meningitis rates were reported in children aged <12 months in Spain (17.8/100,000) and in children aged <2 years in Belgium (16.1/100,000) [3]. Based on an extensive literature review of publications from 1980 to 2005, the overall case fatality rate (CFR) in children aged <5 years with pneumococcal meningitis was 59% (95% CI 27–80%), ranging from 38% (95% CI 32–58%) in Europe to 73% (95% CI 18–94%) in Africa [2]. Mortality and morbidity rates in patients with pneumococcal meningitis vary by age, pneumococcal serotype and geographical location [4, 5].
Untreated bacterial meningitis almost always results in death, and even with optimal treatment death and morbidity can occur [4]. Neurological sequelae are relatively common in survivors of meningitis, particularly after pneumococcal meningitis [4]. Sequelae, such as hearing loss, blindness, seizures, hydrocephalus, developmental delays and motor deficits, have been reported in up to 20% of cases of pneumococcal meningitis in Sweden and in 30% of cases in France, implying a lifetime of considerable disability for those who survive due to a preventable health burden [3, 6]. In France, the mortality rate for bacterial meningitis in the pre-vaccination period was highest in very young children; 13.1%, 14.9% and 9.9% for those aged <1 month, 1 to <2 months and 2 to <12 months, respectively, compared with 6.3% in those aged ≥5 years [7].
Vaccines have played a pivotal role in reducing the burden of bacterial meningitis. Results from clinical trials with the Haemophilus, pneumococcal and meningococcal conjugate vaccines clearly demonstrate their ability to reduce the incidence of invasive disease, including meningitis [8]. After the introduction of the Haemophilus influenzae type b (Hib) conjugate vaccine, there was a substantial decrease in the incidence of Hib meningitis, while the incidences of pneumococcal and meningococcal meningitis remained stable [6].
PCV7 was initially licensed in the US in 2000 and in Europe in 2001; it was introduced into national immunization programs (NIP) in 2000 in the US and in 2006–2008 in many EU countries. It has been shown to have a dramatic effect on vaccine-type invasive pneumococcal disease (VT-IPD), including meningitis, in both vaccination-target populations and also in older children and adults via an indirect effect (herd effect) [9]. The observed increase in non-VT-IPD has not counter-balanced the decrease in VT-IPD, leading to an overall net reduction in all-type IPD [9]. To address the increase in non-VT-IPD, higher valency PCVs have been developed and have replaced PCV7 in most national immunization programs [10].
To obtain a baseline for the measurement of the impact of the higher-valent PCVs on pneumococcal meningitis, we have summarized the data available on the impact of PCV7 on the incidence of all-type and VT-pneumococcal meningitis, in those targeted for vaccination and in those not targeted for vaccination (indirect effect) in North America and Western Europe.
Methods
The data presented here are part of a larger global literature review on the impact of PCV7 on pneumococcal diseases. The search method has been previously described [9]. In summary, on 19 March 2011 we used the following terms to search PubMed: (pneumonia OR “invasive pneumococcal disease” OR IPD OR “otitis media” OR death) AND [(pneumococcal AND conjugate AND vaccin*) OR PCV]. In this paper, we summarize the data from studies that reported the results from the assessment of the impact of PCV7 on all-type and VT-pneumococcal meningitis in Western Europe, and North America (no studies from Australia were found) for all ages that were published between January 2000 and March 2011. Before/after studies were included if the impact data (percentage change in crude or adjusted incidence rates) were provided or could be calculated. The calculation used was [(incidence pre-vaccination − incidence post-vaccination)/incidence pre-vaccination] × 100.
We excluded publications that reported efficacy (i.e., randomized clinical trials), modeling or health economics studies, studies on specific populations (e.g., those with comorbidities such as HIV-positive, patients with sickle-cell disease) and studies for which the denominator was unknown.
Results
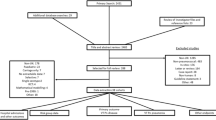
A total of 1,007 publications were identified from the global search, and after two rounds of screening 84 were selected for inclusion in the global literature review (Fig. 1). The main reasons for exclusion were: cohort study with no comparative group (epidemiology data); modeling or cost-effectiveness studies; only specific patient subgroups; review articles and no incidence data or insufficient data for calculating incidence (mainly missing data for the denominator). From these, 17 articles reporting data on the impact of routine childhood PCV vaccination on the incidence of pneumococcal meningitis were analyzed: 6 from North America; 11 from Western Europe (1 Austria, 1 Belgium, 1 Denmark, 1 France, 5 Spain, 1 The Netherlands, 1 England and Wales) and none from Australia [1, 11–26]. The characteristics of these studies are summarized in Table 1. Three of the North American studies used data from the Center for Disease Control (CDC) Active Bacterial Core Surveillance (ABCs) database [23–25]; they used the same pre-PCV vaccination period (1998–1999) but analyzed different post-PCV vaccination periods and populations.
Data on the absolute number of cases were not always available and when available were provided in a variety of ways. For example, overall in France there were 771 cases of pneumococcal meningitis in the pre-vaccination period and 420 in the post-vaccination period; in children aged <2 years there were 181 and 74 cases, respectively [19]. In the UK, in children aged <5 years, there were 107 cases of all-type pneumococcal meningitis in the pre-vaccination period and 49 in the post-vaccination period; there were 82 and 4 cases of VT-pneumococcal meningitis, respectively [21].
Impact on VT-Pneumococcal Meningitis in Vaccine-Eligible Populations
The impact of PCV7 vaccination on VT-pneumococcal meningitis in vaccine-eligible populations ranged from −59.2% (US, in 2001, 1 year after vaccine introduction in the NIP) to −100% (Belgium, 4 years after vaccine introduction in the NIP), with a median of −92.8% (Table 2) [12, 23]. In two other studies in the US, comparing post-vaccination periods of 2004–2005 and 2006–2007 with the same pre-vaccination period as the US study mentioned above, the impact was higher at −92.8% and −97.4%, respectively [23–25].
Impact on All-Type Pneumococcal Meningitis in Vaccine-Eligible Populations
The impact of PCV7 vaccination on all-type pneumococcal meningitis in vaccine-eligible populations ranged from −4.5% (in Spain, in one hospital) to −100% (in another Spanish study in the Basque Country and Navarre) with a median of −47.7% (Table 3) [1, 11–14, 16, 19, 20, 25].
Impact on VT-Pneumococcal Meningitis in Vaccine-Non-Eligible Populations
The impact of PCV7 vaccination on VT-pneumococcal meningitis in vaccine-non-eligible populations ranged from +43.2% (in Spain in subjects aged ≥18 years, in one hospital) to −87.5% (in US in subjects aged ≥65 years), with a median of −67.1% (Table 4) [15, 21, 24, 25].
Impact on All-Type Pneumococcal Meningitis in Other Age Groups
Many studies provided data on all-type pneumococcal meningitis for different age groups, including age groups that could contain both vaccine-eligible and non-vaccine-eligible subjects; these data have been summarized in Table 5. The impact of PCV7 vaccination on all-type pneumococcal meningitis in these other age groups ranged from +137.0% (in Spain in subjects aged ≥18 years, in one hospital) to −76.9% (in another Spanish study in the Basque Country and Navarre, in subjects aged <5 years) (Table 5) [1, 11, 13, 15–22, 24, 25]. A range of 0% (in France) to −37.5% (in the US), with a median of −25.6%, was reported in five studies that provided data for the impact on all-type pneumococcal meningitis in all ages (Table 5).
Evolution of Non-Vaccine-Type Pneumococcal Meningitis in Vaccine-Eligible and Non-Eligible Populations After PCV7 Introduction
Eight studies reported the evolution of the incidence of non-vaccine-type (NVT)-pneumococcal meningitis in different age groups after PCV7 introduction [11, 12, 14, 15, 19, 21, 24, 25]. The incidence of NVT-pneumococcal meningitis was low in the pre-vaccination period, ranging from 0 in children aged 2–4 years in the US to 5.8/100,000 in children aged <2 years in Belgium [12, 25]. In vaccine-eligible populations, three studies reported an increase of +104.2% (from 2.4 to 4.9/100,000) in France, +125.9% (from 5.8 to 13.1/100,000) in Belgium and +272.7% (from 0.77 to 2.87/100,000) in the US, although the absolute incidence remained low [12, 19, 25].
In children aged 5–17 years in one study in the US, there was no change in the incidence of NVT-pneumococcal meningitis, whereas, overall, in all ages there was a significant 60% increase [25]. In vaccine-non-eligible populations, all studies reported an increase in the incidence of NVT-pneumococcal meningitis, ranging from +45% in those aged 5–54 years in the UK to +215.9% (from 0.44 to 1.39/100,000) in all adults in Spain [15, 21]. In those aged ≥65 years, in the US, a decrease of −36.7% (from 0.79 to 0.50/100,000) was reported, whereas in another study in the US, an increase of 27.3% (from 1.1 to 1.4/100,000) was reported [24, 25]. In the UK, for this age group, an increase of 20.0% (0.25–0.30/100,000) was reported [21].
Discussion
There was a substantial reduction in VT-pneumococcal meningitis in both vaccine-eligible and vaccine-non-eligible age groups. While the rate reductions of VT-pneumococcal meningitis were generally similar between different age groups, the pre-PCV7 incidence in children aged <2 years was much higher than in the other age groups so that the relative reduction was greater for this age group. The reduction in VT-pneumococcal meningitis was partially offset by an increase in NVT-pneumococcal meningitis, but generally the increase was not sufficient to prevent an overall reduction; the exceptions to this were generally in the older age groups in studies that were not national (Table 5) [13, 15, 18, 20]. Thus, PCV7 has reduced the incidence of all-type pneumococcal meningitis across all ages, which is a substantial public health benefit.
For the older age groups, the reduction in VT-pneumococcal meningitis is possible evidence of a herd effect. All but one of the studies, in Denmark, reported a reduction in those aged ≥65 years, ranging from 2.6 to 54.2 (Table 5). However, these data should be interpreted with caution due to the low incidence of VT and all-type pneumococcal meningitis in these age groups. In addition, there may be some protection through 23-valent pneumococcal polysaccharide vaccine (PPV23) vaccination in the elderly and adults with comorbidities. However, two studies, one in the US (in adults, with and without PPV23 indications) and one in the UK (in those aged ≥65 years), reported that after PPV23 introduction there was no evidence of a decrease in the incidence of IPD (not just meningitis) caused by serotypes in this vaccine; however, after the introduction of PCV7 in the childhood vaccination programmes, there was a reduction in the incidence of IPD caused by the PCV7 serotypes [27, 28].
Serotypes 19A and 7F have been reported to be responsible for NVT-pneumococcal meningitis in children after the introduction of PCV7 in two recent studies in France and the UK [29, 30]. In addition, in the UK, serotypes 1, 3, 22F and 33F were also found to be responsible for NVT-pneumococcal meningitis [30].
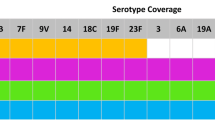
The increased incidence of pneumococcal meningitis caused by non-PCV7 serotypes indicates the need for continued epidemiological surveillance and the development of vaccines with a broader range of protection. The availability of higher valency conjugate vaccines should contribute to the continued reduction in the incidence of pneumococcal meningitis. It was estimated that, in children aged <5 years in the UK in 2009–2010, vaccine serotype coverage for pneumococcal meningitis was 39% for 10-valent PCV (PCV10) and 65% for 13-valent PCV (PCV13) [5]. For the 2004–2005 season in the US, the vaccine serotype coverage was 27.4% for PCV10 and 50.0% for PCV13 [31].
Pneumococcal meningitis is a deadly disease, with an estimated worldwide CFR in children aged <5 years of 59%, compared with 5% for pneumococcal pneumonia, based on an extensive literature review from 1980 to 2005 [2]. The CFR varies in different regions of the world, but even in Europe, for this period, the CFR for pneumococcal meningitis in children <5 years was estimated to be 38%, while in the Americas it was estimated to be 48%. In the studies included in this review, there were only a few that provided meningitis-specific mortality data or CFR; several provided data for pneumococcal IPD-mortality rates [1, 11, 13, 18, 24, 26]. One of the studies in the US reported that the mortality rates fluctuated from 1994 to 1999, before the introduction of PCV7, decreased sharply in 2000 and 2001 and remained relatively stable in 2002–2004, whereas the CFRs fluctuated over this period [1].
The incidences of pneumococcal meningitis, both in the pre-vaccination and post-vaccination eras, were variable between the studies. This variability may be due to several factors including under reporting, differences in reporting methods, differences in blood-culture practices and antibiotic prescribing [3]. This emphasizes the need for well organized, robust, surveillance systems to enable the impact of vaccination on IPD, especially pneumococcal meningitis, to be monitored [2, 3, 32].
The different time periods studied and countries in which the studies were conducted could contribute to the variability, since circulating pneumococcal serotypes show temporal and geographical variations [33–35]. In addition, the pre-vaccination and post-vaccination periods studied, particularly in relation to the date of PCV7 introduction, can have a significant impact. There were also differences in the introduction of PCV7 in terms of catch-up campaigns, which has an effect on the rapidity of impact. In the studies included in this analysis, there were different vaccination schedules (3 + 1 and 2 + 1) used with differing rates of uptake; for example, in the UK, there was rapid uptake after introduction into the NIP with a 2 + 1 schedule, whereas in Spain there was slow uptake after introduction through the private market with a 3 + 1 schedule [18, 21, 36].
One limitation of our review is that in the search strategy we used the term ‘invasive pneumococcal disease’ and not ‘meningitis’ specifically: to assess the impact of this, we retrospectively performed a search which identified four publications, two from Northern France (using the same data source but for different periods) and two from Spain (one of which was in Spanish) [37–40]. Data from a national study in France has already been included [19]. In addition, one other study reporting data from 252 pediatric wards in France could not be included as incidence data were not available for the calculation of the impact [29]. In this study, although the number of cases of pneumococcal meningitis did not decline between before PCV7 introduction (2001–2002) and after (2007–2008), there was a reduction in PCV7-type pneumococcal meningitis from 65.7% to 17.7%. This reduction was greatest in children aged <2 years (from 70.1% to 10.1%). The number of cases of NVT-pneumococcal meningitis increased over this period, mainly 19A in children aged <2 years (8.1–26.9%) and 7F in older children (1.9–10%) [29].
Conclusion
PCV7 has had a significant impact on the overall incidence of pneumococcal meningitis, despite an increase in NVT-pneumococcal meningitis. The higher valency vaccines contain many of the serotypes that are now more commonly responsible for pneumococcal meningitis in both children and adults [5]. With the introduction of PCV13 we can expect to see a reduction in the incidence of pneumococcal meningitis due to the additional six serotypes included, as well as continued protection against IPD due to PCV7 serotypes. Robust surveillance systems will be essential for the evaluation of the impact of PCV13 on all-type pneumococcal meningitis and for monitoring the evolution of NVT-pneumococcal meningitis.
References
Tsai CJ, Griffin MR, Nuorti JP, Grijalva CG. Changing epidemiology of pneumococcal meningitis after the introduction of pneumococcal conjugate vaccine in the United States. Clin Infect Dis. 2008;46(11):1664–72 (Epub 2008/04/25).
O’Brien KL, Wolfson LJ, Watt JP, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374(9693):893–902 (Epub 2009/09/15).
McIntosh ED, Fritzell B, Fletcher MA. Burden of paediatric invasive pneumococcal disease in Europe, 2005. Epidemiol Infect. 2007;135(4):644–56 (Epub 2006/09/09).
Kim KS. Acute bacterial meningitis in infants and children. Lancet Infect Dis. 2010;10(1):32–42 (Epub 2010/02/05).
van Hoek AJ, Andrews N, Waight PA, George R, Miller E. Effect of serotype on focus and mortality of invasive pneumococcal disease: coverage of different vaccines and insight into non-vaccine serotypes. PloS one. 2012;7(7):e39150 (Epub 2012/07/21).
Neuman HB, Wald ER. Bacterial meningitis in childhood at the Children’s Hospital of Pittsburgh: 1988–1998. Clin Pediatr (Phila). 2001;40(11):595–600 (Epub 2002/01/05).
Levy C, Bingen E, Aujard Y, et al. Surveillance network of bacterial meningitis in children, 7 years of survey in France. Arch Pediatr. 2008;15(Suppl 3):S99–104 (Epub 2009/03/14. Observatoire national des meningites bacteriennes de l’enfant en France : resultats de 7 annees d’etude).
Chavez-Bueno S, McCracken GH Jr. Bacterial meningitis in children. Pediatr Clin North Am. 2005;52(3):795–810 (vii. Epub 2005/06/01).
Myint TT, Madhava H, Balmer P, et al. The impact of 7-valent pneumococcal conjugate vaccine on invasive pneumococcal disease: a literature review. Adv Ther. 2013;30(2):127–51 (Epub 2013/02/12).
EUVac.Net. Pneumococcal vaccination (PCV) overview in European countries. 2012. http://www.euvac.net/graphics/euvac/vaccination/pcv.html. Accessed 24 Oct 2012.
Rendi-Wagner P, Paulke-Korinek M, Kundi M, et al. National paediatric immunization program of high risk groups: no effect on the incidence of invasive pneumococcal diseases. Vaccine. 2009;27(30):3963–8 (Epub 2009/04/28).
Hanquet G, Lernout T, Vergison A, et al. Impact of conjugate 7-valent vaccination in Belgium: addressing methodological challenges. Vaccine. 2011;29(16):2856–64 (Epub 2011/02/24).
Harboe ZB, Valentiner-Branth P, Benfield TL, et al. Early effectiveness of heptavalent conjugate pneumococcal vaccination on invasive pneumococcal disease after the introduction in the Danish Childhood Immunization Programme. Vaccine. 2010;28(14):2642–7 (Epub 2010/01/26).
Rodenburg GD, de Greeff SC, Jansen AG, et al. Effects of pneumococcal conjugate vaccine 2 years after its introduction, the Netherlands. Emerg Infect Dis. 2010;16(5):816–23 (Epub 2010/04/23).
Ardanuy C, Tubau F, Pallares R, et al. Epidemiology of invasive pneumococcal disease among adult patients in Barcelona before and after pediatric 7-valent pneumococcal conjugate vaccine introduction, 1997-2007. Clin Infect Dis. 2009;48(1):57–64 (Epub 2008/11/28).
Aristegui J, Bernaola E, Pocheville I, et al. Reduction in pediatric invasive pneumococcal disease in the Basque Country and Navarre, Spain, after introduction of the heptavalent pneumococcal conjugate vaccine. Eur J Clin Microbiol Infect Dis. 2007;26(5):303–10 (Epub 2007/04/26).
Calbo E, Diaz A, Canadell E, et al. Invasive pneumococcal disease among children in a health district of Barcelona: early impact of pneumococcal conjugate vaccine. Clin Microbiol Infect. 2006;12(9):867–72.
Guevara M, Barricarte A, Gil-Setas A, et al. Changing epidemiology of invasive pneumococcal disease following increased coverage with the heptavalent conjugate vaccine in Navarre, Spain. Clin Microbiol Infect. 2009;15(11):1013–9.
Lepoutre A, Varon E, Georges S, Gutmann L, Levy-Bruhl D. Impact of infant pneumococcal vaccination on invasive pneumococcal diseases in France, 2001–2006. Euro Surveill. 2008;13(35). pii:18962 (Epub 2008/09/03).
Munoz-Almagro C, Jordan I, Gene A, Latorre C. Garcia–Garcia JJ, Pallares R. Emergence of invasive pneumococcal disease caused by nonvaccine serotypes in the era of 7-valent conjugate vaccine. Clin Infect Dis. 2008;46(2):174–82 (Epub 2008/01/04).
Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis. 2011;11(10):760–8.
Albrich WC, Baughman W, Schmotzer B, Farley MM. Changing characteristics of invasive pneumococcal disease in Metropolitan Atlanta, Georgia, after introduction of a 7-valent pneumococcal conjugate vaccine. Clin Infect Dis. 2007;44(12):1569–76 (Epub 2007/05/23).
Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348(18):1737–46.
Pilishvili T, Lexau C, Farley MM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201(1):32–41 (Epub 2009/12/02).
Hsu HE, Shutt KA, Moore MR, et al. Effect of pneumococcal conjugate vaccine on pneumococcal meningitis. N Engl J Med. 2009;360(3):244–56 (Epub 2009/01/16).
Hennessy TW, Singleton RJ, Bulkow LR, et al. Impact of heptavalent pneumococcal conjugate vaccine on invasive disease, antimicrobial resistance and colonization in Alaska Natives: progress towards elimination of a health disparity. Vaccine. 2005;23(48–49):5464–73 (Epub 2005/09/29).
Andrews NJ, Waight PA, George RC, Slack MP, Miller E. Impact and effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and Wales. Vaccine. 2012;30(48):6802–8 (Epub 2012/09/25).
Muhammad RD, Oza-Frank R, Zell E, et al. Epidemiology of invasive pneumococcal disease among high-risk adults since the introduction of pneumococcal conjugate vaccine for children. Clin Infect Dis. 2013;56(5):e59–67 (Epub 2012/11/17).
Levy C, Varon E, Bingen E, et al. Pneumococcal meningitis in French children before and after the introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2011;30(2):168–70 (Epub 2011/02/08).
Pichon B, Ladhani SN, Slack MP, et al. Changes in molecular epidemiology of streptococcus pneumoniae causing meningitis following introduction of pneumococcal conjugate vaccination in England and Wales. J Clin Microbiol. 2013;51(3):820–7 (Epub 2012/12/28).
Hsu K, Pelton S, Karumuri S, Heisey-Grove D, Klein J. Population-based surveillance for childhood invasive pneumococcal disease in the era of conjugate vaccine. Pediatr Infect Dis J. 2005;24(1):17–23.
Prymula R, Chlibek R, Ivaskeviciene I, et al. Paediatric pneumococcal disease in Central Europe. Eur J Clin Microbiol Infect Dis. 2011;30(11):1311–20 (Epub 2011/06/15).
Hausdorff W. Invasive pneumococcal disease in children: geographic and temporal variations in incidence and serotype distribution. Eur J Pediatr. 2002;161(2):S135–9.
Hausdorff WP, Bryant J, Kloek C, Paradiso PR, Siber GR. The contribution of specific pneumococcal serogroups to different disease manifestations: implications for conjugate vaccine formulation and use, part II. Clin Infect Dis. 2000;30(1):122–40 (Epub 2000/01/05).
Hausdorff WP, Bryant J, Paradiso PR, Siber GR. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clin Infect Dis. 2000;30(1):100–21 (Epub 2000/01/05).
de Aristegui Fernandez J, Corretger Rauet JM, Garcia Martin F, et al. Pneumococcal disease and its prevention. The heptavalent pneumococcal conjugate vaccine (La enfermedad neumococica y su prevencion. Vacuna neumococica conjugada heptavalente). An Esp Pediatr. 2002;56(1):79–90 (Epub 2002/01/17).
Alexandre C, Dubos F, Courouble C, et al. Rebound in the incidence of pneumococcal meningitis in northern France: effect of serotype replacement. Acta Paediatr. 2010;99(11):1686–90 (Epub 2010/07/16).
Casado-Flores J, Rodrigo C, Aristegui J, Martinon JM, Fenoll A, Mendez C. Decline in pneumococcal meningitis in Spain after introduction of the heptavalent pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2008;27(11):1020–2 (Epub 2008/10/11).
Dubos F, Marechal I, Husson MO, et al. Decline in pneumococcal meningitis after the introduction of the heptavalent-pneumococcal conjugate vaccine in northern France. Arch Dis Child. 2007;92(11):1009–12 (Epub 2007/07/13).
Salleras L, Dominguez A, Ciruela P, Izquierdo C, Borras E, Grupo de Trabajo del Sistema de Notificacion Microbiologica de C. Impact of 7-valent pneumococcal conjugate vaccination in a population with low to intermediate vaccination levels. Enferm Infecc Microbiol Clin. 2009;27(5):275–7 (Epub 2009/04/24. Impacto de la vacuna neumococica conjugada heptavalente en una poblacion con valores bajos-intermedios de vacunacion).
Acknowledgments
All authors are employed by Pfizer who manufactures and commercializes PCV7. EpiConcept performed the literature search, study selection and data extraction; this work was funded by Pfizer. The authors take full responsibility for the interpretation and discussion of the data. Editorial assistance in the preparation of this manuscript was provided by Dr Margaret Haugh, MediCom Consult, funded by Pfizer. Dr Tin Tin Htar is the guarantor for this article, and takes responsibility for the integrity of the work as a whole.
Conflict of interest
Dr Tin Tin Htar, Dr Madhava, Dr Balmer, Dr Christopoulou, Dr Menegas and Dr Bonnet are employed by Pfizer.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Tin Tin Htar , M., Madhava, H., Balmer, P. et al. A Review of the Impact of Pneumococcal Polysaccharide Conjugate Vaccine (7-valent) on Pneumococcal Meningitis. Adv Ther 30, 748–762 (2013). https://doi.org/10.1007/s12325-013-0051-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-013-0051-2