ABSTRACT
Introduction
Pulmonary arterial hypertension (PAH) is associated with poor prognosis despite significant recent advances in its treatment. An intravenous formulation of epoprostenol sodium containing glycine and mannitol (epoprostenol GM; GlaxoSmithKline, London, UK) is widely used to treat PAH. A new formulation of epoprostenol sodium containing arginine and sucrose excipients (epoprostenol AS; Actelion Pharmaceuticals Japan Ltd., Tokyo, Japan) shows better stability at room temperature after preparing diluted solutions. The primary objective of this study was to evaluate the safety and tolerability of switching from epoprostenol GM to epoprostenol AS in Japanese patients with PAH. The authors also evaluated the efficacy and treatment satisfaction after switching formulations.
Methods
This was a two-site, open-label, single-arm, Phase 3b study. Eight adult Japanese PAH patients (seven females) treated with a stable dose of epoprostenol GM for ≥30 days were switched to epoprostenol AS and followed for 12 weeks. Outcomes included safety, changes from baseline to 12 weeks in pulmonary hemodynamic factors (pulmonary vascular resistance, mean pulmonary arterial pressure, and cardiac output), and treatment satisfaction, assessed using the Treatment Satisfaction Questionnaire for Medication (TSQM-9).
Results
The mean (range) age and time since diagnosis of PAH were 48 (25–69) years and 6.2 (0.6–13.9) years, respectively. There were no unexpected safety or tolerability concerns after switching formulations. The epoprostenol dose was maintained after switching formulations. There were no significant changes in pulmonary hemodynamic factors from baseline to week 12. Regarding treatment satisfaction, there was a significant improvement in convenience, which is demonstrated in the score of the domain increased from 51.40 ± 10.19 at baseline to 58.33 ± 12.96 at week 12 (P < 0.05).
Conclusions
Switching from epoprostenol GM to the same dose of epoprostenol AS was well tolerated over 12 weeks of treatment, and pulmonary hemodynamics were maintained. Switching to epoprostenol AS was also associated with improvements in treatment satisfaction (convenience). Clinical Trials: JapicCTI-122017.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Pulmonary arterial hypertension (PAH) is a progressive disease with poor prognosis. It is generally characterized by constriction of pulmonary arteries and vascular remodeling. Right ventricular afterload (right ventricular hypertrophy and enlargement) is increased because of elevated pulmonary arterial pressure and pulmonary vascular resistance, which ultimately leads to right cardiac failure and death [1]. Subjective symptoms of PAH include exertional dyspnea, fatigability, palpitations, chest pain, and syncope [2].
Although the underlying mechanisms of PAH are not fully understood, vascular endothelial abnormalities cause an imbalance between vasoconstricting and vasodilating factors. In this situation, vasoconstricting factors exert a greater influence and increase shear stress. It is generally thought that remodeling of the pulmonary arterial media and narrowing of the pulmonary intravascular lumens due to increased cell proliferation are responsible for this imbalance [3–5].
Prostacyclin (PGI2) agents with various modes of action, endothelin receptor antagonists, and phosphodiesterase-5 inhibitors are available for the treatment of PAH. Oral administration of any of these drugs is recommended for patients with PAH of World Health Organization (WHO) functional Class III or lower. For patients with Class IV PAH, continuous intravenous administration of PGI2 is recommended. Concomitant treatment with several drugs having different modes of action is recommended if the clinical response to monotherapy is inadequate [6–8].
PGI2 is a metabolite of arachidonic acid and plays an important role in maintaining pulmonary vascular homeostasis. It is produced by endocapillary cells, and acts as a potent vasodilator and inhibits platelet aggregation. Accordingly, the PGI2 system plays an important role in the inhibition of vascular smooth muscle cell proliferation and helps to protect the vascular endothelium. A small decline in the function of the PGI2 system leads to an imbalance between vasoconstrictors and vasodilators, and appears to contribute to the development of PAH [9–11].
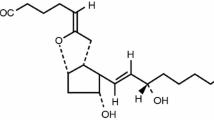
Based on these findings, compounds that target prostacyclin receptors have been developed and are used clinically to treat PAH. One such example is intravenous epoprostenol sodium, a synthetic PGI2 analog, which is prepared as a formulation containing glycine and mannitol as excipients (epoprostenol GM, GlaxoSmithKline, London, UK). Continuous intravenous therapy with epoprostenol GM was reported to improve pulmonary hemodynamic factors, exercise tolerance, and the prognosis of PAH [12]. However, one limitation of this formulation is that the prepared solution is thermally unstable, and needs to be administered within 8 h at room temperature (1–30 °C) or within 24 h if cooled to 2–8 °C using frozen gel packs. Consequently, the medication cassette containing the solution has to be maintained at 2–8 °C using a frozen gel pack for the entire 24-h infusion period [13].
To overcome this limitation, another formulation of epoprostenol sodium, containing arginine and sucrose as excipients (Actelion Pharmaceuticals Japan Ltd., Tokyo, Japan; hereafter, epoprostenol AS), was developed to improve the convenience of using epoprostenol to treat PAH as the new formulation is stable for 24 h at room temperature [14].
To date, however, few studies have examined the safety, potential effects on hemodynamic factors, or treatment satisfaction associated with switching formulations of epoprostenol in patients with PAH [15]. Therefore, the authors performed a 12-week, open-label, Phase 3b study to examine the safety, efficacy, and treatment satisfaction of switching from epoprostenol GM to epoprostenol AS in Japanese patients with PAH. The authors hypothesized that switching from epoprostenol GM to epoprostenol AS would improve treatment satisfaction without increasing the incidence of adverse events or causing deteriorations in pulmonary hemodynamic factors.
METHODS
Ethics
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Subjects
Patients aged ≥20 years at the time of informed consent and who had pulmonary hypertension classified as Group 1 using the Dana Point classification [16,17] for pulmonary hypertension were eligible if they had any of the following: idiopathic pulmonary arterial hypertension (IPAH), heritable pulmonary arterial hypertension (HPAH), PAH associated with drugs and toxins, or PAH associated with connective tissue disease. Only patients who had been treated with epoprostenol GM for ≥3 months before enrollment and at a stable dose for ≥30 days before the start of study treatment were included in the study. Females of childbearing potential had to have a negative serum pregnancy test at screening. They were also required to agree to take monthly urine/serum pregnancy tests and to use reliable contraceptives to avoid pregnancy from the time of the screening visit until 30 days after the end of the study.
Eligible patients were excluded if they met any of the exclusion criteria, such as diagnosis of respiratory or cardiovascular disorder requiring immediate surgery, presence of confirmed or suspected pulmonary vein occlusion, history of myocardial infarction, and resting pulse rate of ≥120 beats/min.
Trial Drug
Epoprostenol AS (Actelion Pharmaceuticals Japan Ltd.) was provided in 10-mL glass vials containing 0.5 mg or 1.5 mg epoprostenol sodium. Epoprostenol AS was dissolved and diluted by adding isotonic sodium chloride solution. At the start of the 12-week treatment period, epoprostenol GM was switched to an equal dose of epoprostenol AS in the hospital. For home therapy, epoprostenol was administered via a central venous catheter by continuous drip infusion using a portable infusion pump.
Study Design
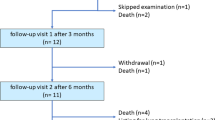
This was a two-site, open-label, single-arm, Phase 3b study. The study consisted of a 2-week pretreatment screening period, a 12-week open-label treatment period (visiting at baseline, week 1, 2, 4, 8, and 12), and a continuous treatment period until marketing of the study drug (visiting every 4 weeks). Pulmonary hemodynamic measurements and variables of clinical laboratory tests were collected at baseline and week 12. Medical interviews and checks for vital signs were performed at each visit. Females of childbearing potential received a pregnancy test every month.
Outcome Measures
Safety/Tolerability
The safety/tolerability endpoints were adverse events occurring during the 12-week treatment phase, together with changes from baseline to week 12 for vital signs (blood pressure and heart rate on the same arm in sitting or supine position), body weight, and abnormal changes from baseline to week 12 for clinical laboratory tests (general biochemistry tests, including thyroid function test and hematology test). Vital signs and body weight were assessed at each visit and clinical laboratory tests were performed at baseline and week 12 (only thyroid function was assessed every month). Adverse events reported during the 12-week evaluation period were coded according to system organ class and terms using the Medical Dictionary for Regulatory Activities/Japanese version. The causality of adverse events in relation to the trial drug was judged by the investigators.
Efficacy Endpoints
The efficacy endpoints were changes in pulmonary hemodynamic factors, WHO functional class, and N-terminal prohormone of brain natriuretic peptide (NT-proBNP) concentrations from baseline (within 60 min before the first dose of epoprostenol AS) to immediately after switching (within 60 min after the first dose of epoprostenol AS) or week 12. Pulmonary hemodynamic factors included systolic pulmonary artery pressure, diastolic pulmonary artery pressure, mean pulmonary artery pressure, pulmonary capillary wedge pressure, cardiac output, mean right atrial pressure, mixed venous oxygen saturation, cardiac index, pulmonary vascular resistance, and pulmonary vascular resistance index. Pulmonary hemodynamics were measured by right heart catheterization, which was performed according to standard local procedures through the internal jugular, subclavian, or femoral vein by a balloon catheter placed into either the right or left pulmonary artery in a sterilized cardiac catheterization laboratory. Cardiac Output (CO) was measured using Fick’s method [18].
Treatment Satisfaction
The abbreviated nine-item Treatment Satisfaction Questionnaire for Medication (TSQM-9), employed for the quality of life assessment, is a validated questionnaire that permits comparisons of patients’ treatment satisfaction across medication types and patient conditions [19]. The changes from baseline to week 12 in treatment satisfaction were assessed using the TSQM-9. This questionnaire includes three items for each of three domains: effectiveness, convenience, and global satisfaction. The scores for each domain range from 0 to 100, where higher scores indicate higher satisfaction on that domain.
Statistical Analysis
This study was an exploratory study. No hypothesis was set and no power considerations were made for this study. Patients who received at least one dose of the study drug were included in the all-treated set for analyses. Patients who had assessable data at baseline and week 12 were included in the analysis of pulmonary hemodynamics. All statistical analyses were considered to be exploratory and the significance level was set at 5% (two-sided). The efficacy variables were summarized descriptively by calculating the mean, standard deviation, standard error, median, 25th and 75th percentiles, minimum and maximum. Changes from baseline were examined using the Wilcoxon signed rank sum test. All analyses were performed using SAS (Version 9.2; SAS Inc., Cary, North Carolina, USA).
RESULTS
Patients
The study was conducted at two Japanese study sites, and started in October 2011. Eight Japanese patients (one male, seven females) with PAH were treated with the study drug and completed the 12-week evaluation period by October 2012. The characteristics of the patients are summarized in Table 1 and Supplemental Table 1. The mean (range) age and time from PAH diagnosis were 47.6 (25–69) years and 6.21 (0.6–13.9) years, respectively. At baseline, seven patients had IPAH and one had HPAH. The WHO functional class was Class I for one patient, Class II for five patients, and Class III for two patients. Epoprostenol AS was started at the same dose of epoprostenol GM, with a mean (range) dose of 40.13 (17.0–61.0) ng/kg/min. The mean (range) duration of exposure to epoprostenol AS during the 12-week treatment period was 86.9 (78.4–91.6) days. There were no dose adjustments in any patient. All of the patients completed the study schedule during the evaluation period. The continuous treatment period was ongoing as of February 2013.
Safety
Adverse events reported during the 12-week evaluation period are summarized in Table 2 according to system organ class and preferred term using the Medical Dictionary for Regulatory Activities/Japanese version. Seven out of eight patients (87.5%) experienced a total of 18 adverse events. Three patients (37.5%) experienced a total of four adverse events that were considered related to the study drug. Two patients (25.0%) experienced serious adverse events: moderate pneumonia and mild device dislocation in one patient each, both of which were not considered related to the study drug. There were no deaths or adverse events leading to treatment discontinuation during the study. Seven out of eight patients (87.5%) experienced mild adverse events and three (37.5%) experienced moderate adverse events; there were no severe adverse events. The most frequent event was nausea, which was reported by two patients. The other adverse events occurred in one patient only. There were no clinically significant changes from baseline to week 12 in blood pressure, heart rate, body weight, or clinical laboratory tests.
Efficacy
Table 3 shows the hemodynamic factors measured within 60 min before (i.e., baseline) and 60 min after the first dose of epoprostenol, as well as the changes between these two times. As shown in Table 3, there were no marked changes in pulmonary hemodynamic parameters from 60 min before to 60 min after the first dose of epoprostenol AS. Wilcoxon signed rank sum tests revealed no significant differences at the 5% level for the changes from baseline. Table 4 presents the hemodynamic factors measured at baseline and at week 12, together with their changes between these times. As shown in Table 4, there were no remarkable changes in pulmonary hemodynamic factors from baseline to week 12. Additionally, Wilcoxon signed rank sum tests revealed no significant differences at the 5% level for the changes from baseline. The WHO functional class was unchanged from baseline to week 12, as one was categorized as Class I, five as Class II, and two as Class III (Table 5). The mean (range) NT-proBNP concentration was 139 (57–240) pg/mL at baseline and 106 (41–243) pg/mL at week 12. The mean (range) change from baseline to week 12 was −–43.3 (−196 to 43) pg/mL, which was not clinically significant (Wilcoxon signed rank sum test: P = 0.5781) (Fig. 1).
Treatment Satisfaction
Table 6 shows the scores for all three domains of the TSQM-9 recorded at baseline and week 12, together with the changes from baseline to week 12. As shown in this table, there were improvements in all three domains during the 12-week treatment period. The improvement in convenience was statistically significant (Wilcoxon signed rank sum test: P = 0.0313).
DISCUSSION
The results of this study demonstrate that patients with PAH can be switched from epoprostenol GM to a new formulation of epoprostenol sodium, epoprostenol AS, with no apparent safety concerns or deteriorations in pulmonary hemodynamic factors. Switching from epoprostenol GM to epoprostenol AS was also associated with an improvement in convenience across three treatment satisfaction domains.
The new formulation of epoprostenol is stable for 24 h at room temperature (1–30 °C) after preparation/dilution in saline, and the prepared solution does not require cooling with a frozen gel pack, which is expected to improve the quality of life of patients, particularly in terms of their daily activities. This expectation is supported by the results of the treatment satisfaction questionnaire, which showed an improvement in convenience. Therefore, patients with PAH treated with conventional formulations of epoprostenol sodium could be switched to epoprostenol AS, a stable formulation at room temperature, and may enhance their quality of life through improvements in treatment convenience.
The outdoor air temperature during the summer in many regions of Japan often exceeds 30 °C, and the temperature around the medication cassette of the diluted solution could be even higher [20]. As part of the risk-management strategy in this study, patients were instructed to use a frozen gel pack during the summer, especially when the environmental temperature exceeded room temperature. In this study, two out of eight patients used a frozen gel pack during the treatment period, but neither of these patients experienced any changes in their overall condition. In addition, the earthquake and tsunami that hit eastern Japan on March 11, 2011, exposed flaws in the systems used to ensure patient safety and maintain a stable supply of medical resources during an emergency [21]. If essential utilities such as electricity and water networks are shut down following a disaster, it is likely that frozen gel packs would not be available, which may be a life-threatening issue for patients with PAH. The authors speculate that epoprostenol AS, which is stable for a longer time at room temperature than the GM formulation, could be valuable for continuing medical treatment during emergencies, and may have a positive impact on the quality of medical care, although additional examination of epoprostenol AS use is required in situations where the temperature exceeds 30 °C. Further studies are needed to evaluate the impact of higher environmental temperatures on the safety/tolerability and efficacy of this formulation, and to accumulate evidence supporting its clinical use.
Some limitations of this study warrant mention. Firstly, the sample size was small, which may prevent detection of small differences in hemodynamic factors or infrequent adverse events. Secondly, as the study was conducted in an open-label manner without a control group (e.g., of patients continuing epoprostenol GM during the 12-week treatment phase), it is possible that a study effect or patient bias contributed to the observed improvements in treatment satisfaction.
CONCLUSION
In conclusion, the present study showed that switching to a new formulation of epoprostenol was associated with an improvement in convenience in relation to treatment satisfaction, without unexpected adverse events or deteriorations in pulmonary hemodynamic factors. Prospective studies in a larger group of patients are needed to confirm the safety of this formulation in long-term clinical use. Epoprostenol AS was approved in February 2013 in Japan as a generic drug with the same potency and effectiveness as the originally approved drug, epoprostenol GM. As intravenous epoprostenol sodium therapy may result in high medical costs, the introduction of cheaper generic drugs may help to reduce medical expenditure for treating PAH.
REFERENCES
Pietra GG, Capron F, Stewart S, et al. Pathologic assessment of vasculopathies in pulmonary hypertension. J Am Coll Cardiol. 2004;43(12 Suppl S):25S–32S.
Rubin LJ. Pathology and pathophysiology of pulmonary arterial hypertension. Am J Cardiol. 1995;75:51A–4A.
Christman BW, McPherson CD, Newman JH, et al. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N Engl J Med. 1992;327:70–5.
Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351:1655–65.
Gatzoulis MA, Alonso-Gonzalez R, Beghetti M. Pulmonary arterial hypertension in paediatric and adult patients with congenital heart disease. Eur Respir Rev. 2009;18:154–61.
Barst RJ, Gibbs JS, Ghofrani HA, et al. Updated evidence-based treatment algorithm in pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54(1 Suppl):S78–84.
Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 2009;30:2493–537.
Hoeper MM, Markevych I, Spiekerkoetter E, Welte T, Niedermeyer J. Goal-oriented treatment and combination therapy for pulmonary arterial hypertension. Eur Respir J. 2005;26:858–63.
Fredenburgh LE, Ma J, Perrella MA. Cyclooxygenase-2 inhibition and hypoxia-induced pulmonary hypertension: effects on pulmonary vascular remodeling and contractility. Trends Cardiovasc Med. 2009;19:31–7.
Tuder RM, Cool CD, Geraci MW, et al. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med. 1999;159:1925–32.
Falcetti E, Hall SM, Phillips PG, et al. Smooth muscle proliferation and role of the prostacyclin (IP) receptor in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2010;182:1161–70.
Barst RJ, Rubin LJ, Long WA, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The Primary Pulmonary Hypertension Study Group. N Engl J Med. 1996;334:296–301.
GlaxoSmithKline. Prescribing Information: Flolan® for Injection (0.5 mg, 1.5 mg). January 2012.
Lambert O, Bandilla D. Stability and preservation of a new formulation of epoprostenol sodium for treatment of pulmonary arterial hypertension. Drug Des Dev Ther. 2012;6:235–44.
Sitbon O, Delcroix M, Bergot E, et al. EPITOME-2, An open-label study evaluating a new formulation of epoprostenol sodium in pulmonary arterial hypertension patients switched from Flolan®. Am J Respir Crit Care Med. 2012; 185 A2500.
Simonneau G. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004;43:5S–12S.
Simonneau G. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43–54.
Acierno LJ. Adolph Fick: mathematician, physicist, physiologist. Clin Cardiol. 2000;23:390–1.
Bharmal M, Payne K, Atkinson MJ, Desrosiers MP, Morisky DE, Gemmen E. Validation of an abbreviated treatment satisfaction questionnaires for medication (TSQM-9) among patients on antihypertensive medications. Health Qual Life Outcomes. 2009;7:36.
Tamura Y, Nakajima Y, Ozeki Y, et al. Temperature variations around medication cassette and carry bag in routine use of epoprostenol administration in healthy volunteers. PLoS ONE. 2012;7:e52216.
Tamura Y, Fukuda K. Earthquake in Japan. Lancet. 2011;377:1652.
Acknowledgments
The study and article processing charges were funded and supported by Actelion Pharmaceuticals Japan Ltd. The authors would like to thank Nicholas D. Smith, PhD, an employee of Edanz, for editorial support in the preparation of this manuscript, which was funded by Actelion Pharmaceuticals Japan Ltd. Dr Yuichi Tamura is the guarantor for this article, and takes responsibility for the integrity of the work as a whole.
Conflict of interest
Yuichi Tamura declares no conflict of interest. Tomohiko Ono declares no conflict of interest. Keiichi Fukuda declares no conflict of interest. Toru Satoh declares no conflict of interest. Shigetake Sasayama declares no conflict of interest.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Tamura, Y., Ono, T., Fukuda, K. et al. Evaluation of a New Formulation of Epoprostenol Sodium in Japanese Patients with Pulmonary Arterial Hypertension (EPITOME4). Adv Ther 30, 459–471 (2013). https://doi.org/10.1007/s12325-013-0029-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-013-0029-0