Abstract
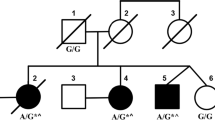
Autosomal recessive spinocerebellar ataxia 13 (SCAR13) is a neurological disease characterized by psychomotor delay, mild to profound intellectual disability with poor or absent language, nystagmus, stance ataxia, and, if walking is acquired, gait ataxia. Epilepsy and polyneuropathy have also been documented in some patients. Cerebellar atrophy and/or ventriculomegaly may be present on brain MRI. SCAR13 is caused by pathogenic variants in the GRM1 gene encoding the metabotropic receptor of glutamate type 1 (mGlur1), which is highly expressed in Purkinje cerebellar cells, where it plays a fundamental role in cerebellar development. Here we discuss the case of an 8-year-old patient who presented with a severe neurodevelopmental disorder with balance disturbance, absence of independent walking, absence of language, diffuse hypotonia, mild nystagmus, and mild dysphagia. Whole-exome sequencing revealed a compound heterozygosity for two likely pathogenic variants in the GRM1 gene, responsible for the patient’s phenotype, and made it possible to diagnose autosomal recessive spinocerebellar ataxia SCAR13. The detected (novel) variants appear to be causative of a particularly severe picture with regard to neurodevelopment, in the context of the typical neurological signs of spinocerebellar ataxia.
Similar content being viewed by others
Data Availability
The data used for this paper will be made available by the corresponding author upon reasonable request.
References
Kapfhammer JP, Shimobayashi E. Viewpoint: spinocerebellar ataxias as diseases of Purkinje cell dysfunction rather than Purkinje cell loss. Front Mol Neurosci. 2023;22(16):1182431. https://doi.org/10.3389/fnmol.2023.1182431.
Klockgether T, Mariotti C, Paulson HL. Spinocerebellar ataxia. Nat Rev Dis Primers. 2019;5(1):24. https://doi.org/10.1038/s41572-019-0074-3.
Müller U. Spinocerebellar ataxias (SCAs) caused by common mutations. Neurogenetics. 2021;22(4):235–50. https://doi.org/10.1007/s10048-021-00662-5.
Bhandari J, Thada PK, Samanta D. Spinocerebellar ataxia. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
Yousaf H, Fatima A, Ali Z, Baig SM, Toft M, Iqbal Z. A novel nonsense variant in GRM1 causes autosomal recessive spinocerebellar ataxia 13 in a consanguineous Pakistani family. Genes (Basel). 2022;13(9):1667. https://doi.org/10.3390/genes13091667.
Protasova MS, Andreeva TV, Klyushnikov SA, Illarioshkin SN, Rogaev EI. Genetic variant in GRM1 underlies congenital cerebellar ataxia with no obvious intellectual disability. Int J Mol Sci. 2023;24(2):1551. https://doi.org/10.3390/ijms24021551.
Kapushesky M, Emam I, Holloway E, Kurnosov P, Zorin A, Malone J, Rustici G, Williams E, Parkinson H, Brazma A. Gene expression Atlas at the European Bioinformatics Institute. Nucleic Acids Res. 2010;38:D690–8.
Lindsay SJ, Xu Y, Lisgo SN, Harkin LF, Copp AJ, Gerrelli D, Clowry GJ, Talbot A, Keogh MJ, Coxhead J, et al. HDBR Expression: a unique resource for global and individual gene expression studies during early human brain development. Front Neuroanat. 2016;10:86.
Su LD, Wang N, Han J, Shen Y. Group 1 metabotropic glutamate receptors in neurological and psychiatric diseases: mechanisms and prospective. Neuroscientist. 2022;28(5):453–68. https://doi.org/10.1177/10738584211021018.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL, ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–24. https://doi.org/10.1038/gim.2015.30.
Tavtigian SV, Harrison SM, Boucher KM, Biesecker LG. Fitting a naturally scaled point system to the ACMG/AMP variant classification guidelines. Hum Mutat. 2020;41(10):1734–7. https://doi.org/10.1002/humu.24088.
Guergueltcheva V, Azmanov DN, Angelicheva D, Smith KR, Chamova T, Florez L, Bynevelt M, Nguyen T, Cherninkova S, Bojinova V, Kaprelyan A, Angelova L, Morar B, Chandler D, Kaneva R, Bahlo M, Tournev I, Kalaydjieva L. Autosomal-recessive congenital cerebellar ataxia is caused by mutations in metabotropic glutamate receptor 1. Am J Hum Genet. 2012;91(3):553–64. https://doi.org/10.1016/j.ajhg.2012.07.019.
Davarniya B, Hu H, Kahrizi K, Musante L, Fattahi Z, Hosseini M, Maqsoud F, Farajollahi R, Wienker TF, Ropers HH, Najmabadi H. The role of a novel TRMT1 gene mutation and rare GRM1 gene defect in intellectual disability in two Azeri families. PLoS ONE. 2015;10(8):e0129631. https://doi.org/10.1371/journal.pone.0129631.
Cabet S, Putoux A, Carneiro M, Labalme A, Sanlaville D, Guibaud L, Lesca G. A novel truncating variant p.(Arg297*) in the GRM1 gene causing autosomal-recessive cerebellar ataxia with juvenile-onset. Eur J Med Genet. 2019;62(10):103726. https://doi.org/10.1016/j.ejmg.2019.103726.
Auerbach BD, Osterweil EK, Bear MF. Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature. 2011;480(7375):63–8. https://doi.org/10.1038/nature10658.
Mercer AA, Palarz KJ, Tabatadze N, Woolley CS, Raman IM. Sex differences in cerebellar synaptic transmission and sex-specific responses to autism-linked Gabrb3 mutations in mice. Elife. 2016;14(5):e07596. https://doi.org/10.7554/eLife.07596.
Seese RR, Maske AR, Lynch G, Gall CM. Long-term memory deficits are associated with elevated synaptic ERK1/2 activation and reversed by mGluR5 antagonism in an animal model of autism. Neuropsychopharmacology. 2014;39(7):1664–73. https://doi.org/10.1038/npp.2014.13.
Wang X, Bey AL, Katz BM, Badea A, Kim N, David LK, Duffney LJ, Kumar S, Mague SD, Hulbert SW, Dutta N, Hayrapetyan V, Yu C, Gaidis E, Zhao S, Ding JD, Xu Q, Chung L, Rodriguiz RM, Wang F, Weinberg RJ, Wetsel WC, Dzirasa K, Yin H, Jiang YH. Altered mGluR5-Homer scaffolds and corticostriatal connectivity in a Shank3 complete knockout model of autism. Nat Commun. 2016;10(7):11459. https://doi.org/10.1038/ncomms11459.
Wenger TL, Kao C, McDonald-McGinn DM, Zackai EH, Bailey A, Schultz RT, Morrow BE, Emanuel BS, Hakonarson H. The role of mGluR copy number variation in genetic and environmental forms of syndromic autism spectrum disorder. Sci Rep. 2016;19(6):19372. https://doi.org/10.1038/srep19372.
Brašić JR, Nandi A, Russell DS, Jennings D, Barret O, Martin SD, Slifer K, Sedlak T, Seibyl JP, Wong DF, Budimirovic DB. Cerebral expression of metabotropic glutamate receptor subtype 5 in idiopathic autism spectrum disorder and fragile X syndrome: a pilot study. Int J Mol Sci. 2021;22(6):2863. https://doi.org/10.3390/ijms22062863.
Bourgeron T. From the genetic architecture to synaptic plasticity in autism spectrum disorder. Nat Rev Neurosci. 2015;16(9):551–63. https://doi.org/10.1038/nrn3992.
Ramsakha N, Ojha P, Pal S, Routh S, Citri A, Bhattacharyya S. A vital role for PICK1 in the differential regulation of metabotropic glutamate receptor internalization and synaptic AMPA receptor endocytosis. J Biol Chem. 2023;299(6):104837. https://doi.org/10.1016/j.jbc.2023.104837.
Acknowledgements
This work has been generated within the European Reference Network on Rare Congenital Malformations and Rare Intellectual Disability (ERN-ITHACA) (EU Framework Partnership Agreement ID: 3HP-HP-FPA ERN-01-2016/739516). The authors also wish to thank the patient’s family members for their cooperation in providing the medical data for this publication.
Author information
Authors and Affiliations
Contributions
Conceptualization, C.A.C., G.P.; clinical data collection and data curation, C.A.C., G.P., G.T., G.S., M.G., C.S., S.R., C.F.; writing—original draft preparation, C.A.C., G.P.; writing—review and editing, C.A.C., G.P., G.T., C.S., C.F., C.D., G.S., S.G.C., L.G.; supervision, L.G., C.F. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
The authors declare that this paper complies with internationally-accepted standards for research practice and reporting. Written informed consent to participate and written consent to publish was obtained from the patient’s parents for the publication of this report.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cesaroni, C.A., Pisanò, G., Trimarchi, G. et al. Severe Neurodevelopmental Disorder in Autosomal Recessive Spinocerebellar Ataxia 13 (SCAR13) Caused by Two Novel Frameshift Variants in GRM1. Cerebellum (2023). https://doi.org/10.1007/s12311-023-01617-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s12311-023-01617-2