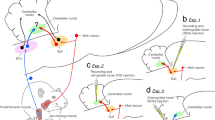
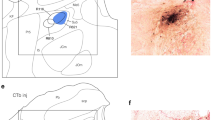
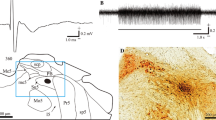
Abstract
Proprioceptive sensory information from muscle spindles is essential for the regulation of motor functions. However, little is known about the motor control regions in the cerebellar cortex that receive proprioceptive signals from muscle spindles distributed throughout the body, including the orofacial muscles. Therefore, in this study, we investigated the pattern of projections in the rat cerebellar cortex derived from the supratrigeminal nucleus (Su5), which conveys orofacial proprioceptive information from jaw-closing muscle spindles (JCMSs). Injections of an anterograde tracer into the Su5 revealed that many bilateral axon terminals (rosettes) were distributed in the granular layer of the cerebellar cortex (including the simple lobule B, crus II and flocculus) in a various sized, multiple patchy pattern. We could also detect JCMS proprioceptive signals in these cerebellar cortical regions, revealing for the first time that they receive muscle proprioceptive inputs in rats. Retrograde tracer injections confirmed that the Su5 directly sends outputs to the cerebellar cortical areas. Furthermore, we injected an anterograde tracer into the external cuneate nucleus (ECu), which receives proprioceptive signals from the forelimb and neck muscle spindles, to distinguish between the Su5- and ECu-derived projections in the cerebellar cortex. The labeled terminals from the ECu were distributed predominantly in the vermis of the cerebellar cortex. Almost no overlap was seen in the terminal distributions of the Su5 and ECu projections. Our findings demonstrate that the rat cerebellar cortex receives orofacial proprioceptive input that is processed differently from the proprioceptive signals from the other regions of the body.








Similar content being viewed by others
Data Availability
All data and materials are available upon request.
Code Availability
Not applicable.
Abbreviations
- I–X:
-
Lobules I–X
- 5C:
-
Caudal subnucleus of the trigeminal spinal nucleus
- 5I:
-
Interpolar subnucleus of the trigeminal spinal nucleus
- 7n:
-
Facial nerve
- 10:
-
Dorsal motor nucleus of vagus
- 12 :
-
Hypoglossal nucleus
- AP:
-
Area postrema
- BDA:
-
Biotinylated dextran amine
- BPn:
-
Basilar pontine nuclei
- Cop:
-
Copula pyramidis
- Crus I:
-
Crus I of the ansiform lobule
- Crus II:
-
Crus II of the ansiform lobule
- CTb:
-
Cholera toxin B subunit
- Cu:
-
Cuneate nucleus
- cu:
-
Cuneate fasciculus
- ECu:
-
External cuneate nucleus
- FG:
-
Fluorogold
- FL:
-
Flocculus
- Gr:
-
Gracile nucleus
- H II–V:
-
Hemisphere of lobules II–V
- I5:
-
Intertrigeminal region
- JCMS:
-
Jaw-closing muscle spindle
- KF:
-
Kölliker-Fuse nucleus
- LC:
-
Locus coeruleus
- LRt:
-
Lateral reticular nucleus
- M1:
-
Primary motor cortex
- Me5:
-
Trigeminal mesencephalic nucleus
- me5:
-
Trigeminal mesencephalic tract
- Mo5:
-
Trigeminal motor nucleus
- Pa5:
-
Paratrigeminal nucleus
- PB:
-
Phosphate buffer
- Pb:
-
Parabrachial nucleus
- PFL:
-
Paraflocculus
- PM:
-
Paramedian lobule
- Pr5:
-
Trigeminal principal nucleus
- RtTg:
-
Reticulotegmental nucleus of the pons
- scp:
-
Superior cerebellar peduncle
- Sim A:
-
Simple lobule A
- Sim B:
-
Simple lobule B
- Sol:
-
Solitary tract nucleus
- sp5:
-
Spinal trigeminal tract
- Su5:
-
Supratrigeminal nucleus
- Ves:
-
Vestibular nuclei
- VPM:
-
Ventral posteromedial thalamic nucleus
References
Eccles JC, Ito M, Szentágothai J. The cerebellum as a neuronal machine. Berlin-Heidelberg-New York: Springer-Verlag; 1967. p. 227–61.
Palay SL, Chan-Palay V. Cerebellar cortex: Cytology and organization. Berlin: Springer-Verlag; 1974.
Ito M. The Cerebellum and Neural Control. New York: Raven Press; 1984.
Quy PN, Fujita H, Sakamoto Y, Na J, Sugihara I. Projection patterns of single mossy fiber axons originating from the dorsal column nuclei mapped on the aldolase C compartments in the rat cerebellar cortex. J Comp Neurol. 2011;519:874–99.
Dubner R, Sessle BJ, Storey AT. The neural basis of oral and facial function. New York: Plenum Press; 1978.
Gould BB. Organization of afferents from the brain stem nuclei to the cerebellar cortex in the cat. Adv Anat Embryol Cell Biol. 1980;62:1–90.
Matsushita M, Ikeda M, Okado N. The cells of origin of the trigeminothalamic, trigeminospinal and trigeminocerebellar projections in the cat. Neuroscience. 1982;7:1439–54.
Donga R, Dessem D. An unrelayed projection of jaw-muscle spindle afferents to the cerebellum. Brain Res. 1993;626:347–50.
Dessem D, Donga R, Luo P. Primary- and secondary-like jaw-muscle spindle afferents have characteristic topographic distributions. J Neurophysiol. 1997;77:2925–44.
Nomura S, Mizuno N. Differential distribution of cell bodies and central axons of mesencephalic trigeminal nucleus neurons supplying the jaw-closing muscles and periodontal tissue: a transganglionic tracer study in the cat. Brain Res. 1985;359:311–9.
Kishimoto H, Bae YC, Yoshida A, Moritani M, Takemura M, Nakagawa S, Nagase Y, Wada T, Sessle BJ, Shigenaga Y. Central distribution of synaptic contacts of primary and secondary jaw muscle spindle afferents in the trigeminal motor nucleus of the cat. J Comp Neurol. 1998;391:50–63.
Yoshida A, Mukai N, Moritani M, Nagase Y, Hirose Y, Honma S, Fukami H, Takagi K, Matsuya T, Shigenaga Y. Physiologic and morphologic properties of motoneurons and spindle afferents innervating the temporal muscle in the cat. J Comp Neurol. 1999;406:29–50.
Lingenhöhl K, Friauf E. Sensory neurons and motoneurons of the jaw-closing reflex pathway in rats: a combined morphological and physiological study using the intracellular horseradish peroxidase technique. Exp Brain Res. 1991;83:385–96.
Yoshida A, Tsuru K, Mitsuhiro Y, Otani K, Shigenaga Y. Morphology of masticatory motoneurons stained intracellularly with horseradish peroxidase. Brain Res. 1987;416:393–401.
Shigenaga Y, Yoshida A, Tsuru K, Mitsuhiro Y, Otani K, Cao CQ. Physiological and morphological characteristics of cat masticatory motoneurons–intracellular injection of HRP. Brain Res. 1988;461:238–56.
Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th ed. Sydney: Academic Press; 1998.
Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 7th ed. Sydney: Academic Press; 2014.
Fujio T, Sato F, Tachibana Y, Kato T, Tomita A, Higashiyama K, Ono T, Maeda Y, Yoshida A. Revisiting the supratrigeminal nucleus in the rat. Neuroscience. 2016;324:307–20.
Sato F, Kado S, Tsutsumi Y, Tachibana Y, Ikenoue E, Furuta T, Uchino K, Bae YC, Uzawa N, Yoshida A. Ascending projection of jaw-closing muscle-proprioception to the intralaminar thalamic nuclei in rats. Brain Res. 2020;1739:146830.
Yoshida A, Fujio T, Sato F, Ali MS, Haque T, Ohara H, Moritani M, Kato T, Dostrovsky JO, Tachibana Y. Orofacial proprioceptive thalamus of the rat. Brain Struct Funct. 2017;222:2655–69.
Haque T, Yamamoto S, Masuda Y, Kato T, Sato F, Uchino K, Oka A, Nakamura M, Takeda R, Ono T, Kogo M, Yoshida A. Thalamic afferent and efferent connectivity to cerebral cortical areas with direct projections to identified subgroups of trigeminal premotoneurons in the rat. Brain Res. 2010;1346:69–82.
Iida C, Oka A, Moritani M, Kato T, Haque T, Sato F, Nakamura M, Uchino K, Seki S, Bae YC, Takada K, Yoshida A. Corticofugal direct projections to primary afferent neurons in the trigeminal mesencephalic nucleus of rats. Neuroscience. 2010;169:1739–57.
Campbell SK, Parker TD, Welker W. Somatotopic organization of the external cuneate nucleus in albino rats. Brain Res. 1974;77:1–23.
Uemura Y, Haque T, Sato F, Tsutsumi Y, Ohara H, Oka A, Furuta T, Bae YC, Yamashiro T, Tachibana Y, Yoshida A. Proprioceptive thalamus receiving forelimb and neck muscle spindle inputs via the external cuneate nucleus in the rat. Brain Struct Funct. 2020;225:2177–92.
Tsutsumi Y, Tachibana Y, Sato F, Furuta T, Ohara H, Tomita A, Fujita M, Moritani M, Yoshida A. Cortical and subcortical projections from granular insular cortex receiving orofacial proprioception. Neuroscience. 2018;388:317–29.
Sato F, Uemura Y, Kanno C, Tsutsumi Y, Tomita A, Oka A, Kato T, Uchino K, Murakami J, Haque T, Tachibana Y, Yoshida A. Thalamo-insular pathway conveying orofacial muscle proprioception in the rat. Neuroscience. 2017;365:158–78.
Tsutsumi Y, Mizuno Y, Haque T, Sato F, Furuta T, Oka A, Moritani M, Bae YC, Yamashiro T, Tachibana Y, Yoshida A. Widespread corticopetal projections from the oval paracentral nucleus of the intralaminar thalamic nuclei conveying orofacial proprioception in rats. Brain Struct Funct. 2021;226:1115–33.
Buisseret-Delmas C. Sagittal organization of the olivocerebellonuclear pathway in the rat. I. Connections with the nucleus fastigii and the nucleus vestibularis lateralis. Neurosci Res. 1988;5:475–93.
Swanson LW. Brain maps: structure of the rat brain. Amsterdam: Elsevier; 1992.
Taylor A. Neurophysiology of the Jaws and Teeth. London: Macmillan Press; 1990.
Kobayashi Y. Distribution and size of cerebellar and thalamic projection neurons in the trigeminal principal sensory nucleus and adjacent nuclei in the rat. Kaibogaku Zasshi. 1995;70:156–71.
Azzena GB, Desole C, Palmieri G. Cerebellar projections of the masticatory and extraocular muscle proprioception. Exp Neurol. 1970;27:151–61.
Shambes GM, Gibson JM, Welker W. Fractured somatotopy in granule cell tactile areas of rat cerebellar hemispheres revealed by micromapping. Brain Behav Evol. 1978;15:94–140.
Cody FW, Richardson HC. Mossy and climbing fibre mediated responses evoked in the cerebellar cortex of the cat by trigeminal afferent stimulation. J Physiol. 1979;287:1–14.
Rosén I, Sjölund B. Organization of group I activated cells in the main and external cuneate nuclei of the cat: identification of muscle receptors. Exp Brain Res. 1973;16:221–37.
Dykes RW, Rasmusson DD, Sretavan D, Rehman NB. Submodality segregation and receptive-field sequences in cuneate, gracile, and external cuneate nuclei of the cat. J Neurophysiol. 1982;47:389–416.
Jasmin L, Courville J. Distribution of external cuneate nucleus afferents to the cerebellum: II. Topographical distribution and zonal pattern–an experimental study with radioactive tracers in the cat. J Comp Neurol. 1987;261:497–514.
Na J, Sugihara I, Shinoda Y. The entire trajectories of single pontocerebellar axons and their lobular and longitudinal terminal distribution patterns in multiple aldolase C-positive compartments of the rat cerebellar cortex. J Comp Neurol. 2019;527:2488–511.
Wu HS, Sugihara I, Shinoda Y. Projection patterns of single mossy fibers originating from the lateral reticular nucleus in the rat cerebellar cortex and nuclei. J Comp Neurol. 1999;411:97–118.
Serapide MF, Parenti R, Pantò MR, Zappalà A, Cicirata F. Multiple zonal projections of the nucleus reticularis tegmenti pontis to the cerebellar cortex of the rat. Eur J Neurosci. 2002;15:1854–8.
Blanks RH, Precht W, Torigoe Y. Afferent projections to the cerebellar flocculus in the pigmented rat demonstrated by retrograde transport of horseradish peroxidase. Exp Brain Res. 1983;52:293–306.
Ruigrok TJH. Collateralization of climbing and mossy fibers projecting to the nodulus and flocculus of the rat cerebellum. J Comp Neurol. 2003;466:278–98.
Mihailoff GA, Burne RA, Azizi SA, Norell G, Woodward DJ. The pontocerebellar system in the rat: an HRP study. II Hemispheral components J Comp Neurol. 1981;197:559–77.
Mihailoff GA. Intra- and interhemispheric collateral branching in the rat pontocerebellar system, a fluorescence double-label study. Neuroscience. 1983;10:141–60.
Hrycyshyn AH, Flumerfelt BA, Anderson WA. A horseradish peroxidase study of the projections from the lateral reticular nucleus to the cerebellum in the rat. Anat Embryol (Berl). 1982;165:1–18.
Yoshida A, Inoue M, Sato F, Morita Y, Tsutsumi Y, Furuta T, Uchino K, Akhter F, Bae YC, Tachibana Y, Inoue T. Efferent and afferent connections of supratrigeminal neurons conveying orofacial muscle proprioception in rats. Brain Struct Funct. 2022;227:111–29.
Crandall WF, Keller EL. Visual and oculomotor signals in nucleus reticularis tegmenti pontis in alert monkey. J Neurophysiol. 1985;54:1326–45.
Torigoe Y, Blanks RH, Precht W. Anatomical studies on the nucleus reticularis tegmenti pontis in the pigmented rat. I. Cytoarchitecture, topography, and cerebral cortical afferents. J Comp Neurol. 1986;243:71–87.
Suzuki DA, Yamada T, Yee RD. Smooth-pursuit eye-movement-related neuronal activity in macaque nucleus reticularis tegmenti pontis. J Neurophysiol. 2003;89:2146–58.
Thier P, Möck M. The oculomotor role of the pontine nuclei and the nucleus reticularis tegmenti pontis. Prog Brain Res. 2006;151:293–320.
Wiesendanger R, Wiesendanger M. The corticopontine system in the rat. II. The projection pattern J Comp Neurol. 1982;208:227–38.
Mihailoff GA, Lee H, Watt CB, Yates R. Projections to the basilar pontine nuclei from face sensory and motor regions of the cerebral cortex in the rat. J Comp Neurol. 1985;237:251–63.
Mihailoff GA, Kosinski RJ, Azizi SA, Border BG. Survey of noncortical afferent projections to the basilar pontine nuclei: a retrograde tracing study in the rat. J Comp Neurol. 1989;282:617–43.
Kosinski RJ, Azizi SA, Mihailoff GA. Convergence of cortico- and cuneopontine projections onto components of the pontocerebellar system in the rat: an anatomical and electrophysiological study. Exp Brain Res. 1988;71:541–56.
Watson CR, Switzer RC 3rd. Trigeminal projections to cerebellar tactile areas in the rat-origin mainly from n. interpolaris and n. principalis. Neurosci Lett. 1978;10:77–82.
Huerta MF, Frankfurter A, Harting JK. Studies of the principal sensory and spinal trigeminal nuclei of the rat: projections to the superior colliculus, inferior olive, and cerebellum. J Comp Neurol. 1983;220:147–67.
Falls WM, Rice RE, VanWagner JP. The dorsomedial portion of trigeminal nucleus oralis (Vo) in the rat: cytology and projections to the cerebellum. Somatosens Res. 1985;3:89–118.
Phelan KD, Falls WM. A comparison of the distribution and morphology of thalamic, cerebellar and spinal projection neurons in rat trigeminal nucleus interpolaris. Neuroscience. 1991;40:497–511.
Ikeda M. Projections from the spinal and the principal sensory nuclei of the trigeminal nerve to the cerebellar cortex in the cat, as studied by retrograde transport of horseradish peroxidase. J Comp Neurol. 1979;184:567–86.
Somana R, Kotchabhakdi N, Walberg F. Cerebellar afferents from the trigeminal sensory nuclei in the cat. Exp Brain Res. 1980;38:57–64.
Joseph JW, Shambes GM, Gibson JM, Welker W. Tactile projections to granule cells in caudal vermis of the rat’s cerebellum. Brain Behav Evol. 1978;15:141–9.
Shambes GM, Beermann DH, Welker W. Multiple tactile areas in cerebellar cortex: another patchy cutaneous projection to granule cell columns in rats. Brain Res. 1978;157:123–8.
Welker W, Shambes GM. Tactile cutaneous representation in cerebellar granule cell layer of the opossum. Didelphis virginiana Brain Behav Evol. 1985;27:57–79.
Sherrington CS. The integrative action of the nervous system. London: Yale University Press; 1906.
Manni E, Petrosini L. Luciani’s work on the cerebellum a century later. Trends Neurosci. 1997;20:112–6.
Manni E, Petrosini L. A century of cerebellar somatotopy: a debated representation. Nat Rev Neurosci. 2004;5:241–9.
Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003;23:8432–44.
Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BTT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:2322–45.
Barnes GR. Visual-vestibular interaction in the control of head and eye movement: the role of visual feedback and predictive mechanisms. Prog Neurobiol. 1993;41:435–72.
Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16:367–78.
Koziol LF, Budding D, Andreasen N, D’Arrigo S, Bulgheroni S, Imamizu H, Ito M, Manto M, Marvel C, Parker K, Pezzulo G, Ramnani N, Riva D, Schmahmann JD, Vandervert L, Yamazaki T. Consensus paper: the cerebellum’s role in movement and cognition. Cerebellum. 2014;13:151–77.
Sasaki K, Oka S, Jinnai K, Yasuda T. Mossy fibre and climbing fibre responses produced in the cerebeller cortex by stimulation of the cerebral cortex in monkeys. Exp Brain Res. 1977;29:419–28.
Marvel CL, Desmond JE. The contributions of cerebro-cerebellar circuitry to executive verbal working memory. Cortex. 2010;46:880–95.
Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44:489–501.
Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46:831–44.
Turner BM, Paradiso S, Marvel CL, Pierson R, Ponto LLB, Hichwa RD, Robinson RG. The cerebellum and emotional experience. Neuropsychologia. 2007;45:1331–41.
Brodal P, Bjaalie JG. Salient anatomic features of the cortico-ponto-cerebellar pathway. Prog Brain Res. 1997;114:227–49.
Funding
This work was supported by Grants-in-Aid for Scientific Research of the Japan Society for the Promotion of Science (DC-1 21J21394 to Y.Ts.; 20K09888 to F.S.; 18KK0259 and 21K09814 to A.Y.).
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript. AY and YTs conceptualized the hypothesis, designed and supervised the experiments, and directed the data analysis. YTs and FS carried out the experiments and data analysis. MM and KU helped with the experiments and data analysis. AY, YTs, TF, YCB, TK and YTa finalized the figures and text.
Corresponding author
Ethics declarations
Ethical Approval and Consent to Participate
Detailed protocols for the care and use of laboratory animals were approved by the animal ethics committees of the Osaka University Graduate School of Dentistry.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tsutsumi, Y., Sato, F., Furuta, T. et al. The Cerebellar Cortex Receives Orofacial Proprioceptive Signals from the Supratrigeminal Nucleus via the Mossy Fiber Pathway in Rats. Cerebellum 22, 663–679 (2023). https://doi.org/10.1007/s12311-022-01434-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-022-01434-z