Abstract
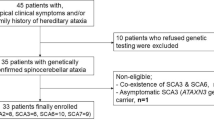
While dynamic ocular motor abnormalities (e.g., gaze-evoked nystagmus (GEN), low optokinetic nystagmus (OKN), pursuit and vestibulo-ocular reflex (VOR) gains, and dysmetric saccades) have been shown to be potential biomarkers in spinocerebellar ataxia type 3 (SCA3), the value of static abnormalities (e.g., convergent [esodeviation] and divergent strabismus [exodeviation]) is unknown. Moreover, studies on dynamic abnormalities in SCA3 usually do not take into account the existence of potential abduction-adduction asymmetries in patients with degenerative ataxia. Thirty-eight patients with genetically confirmed SCA3 (24 females; mean age ± SD, 49.8± 12.2 years) and 22 healthy controls (12 females, p = 0.589; mean age ± SD, 50.7± 12.5 years, p = 0.651) underwent clinical and video-oculographic assessment. A p value < 0.002 (between- and within-group analyses) and < 0.001 (correlation analysis) was considered significant. Patients showed larger esodeviation at distance (p < 0.001), became more esodeviated in lateral gaze (p < 0.001), and their near exodeviation correlated with scale for the assessment and rating of ataxia (SARA) score (p = 0.004). Pursuit, OKN, and VOR gains were lower in patients, both for their adducting and abducting components (p < 0.001). Saccades showed higher velocities (p < 0.001), abducting saccades showed lower amplitude (p < 0.001), and adducting saccades tended to show greater vertical bias (p = 0.018) in patients. Abducting saccades showed relatively lower velocity (p < 0.001) and lower amplitude (p = 0.015) than abducting saccades within patients. All dynamic ocular motor abnormalities except saccades correlated with SARA score, CAG repeat number, and/or disease duration (p < 0.001). Static and dynamic ocular motor abnormalities are potential biomarkers in SCA3. SCA3 studies using saccades should take into account the existence of potential abduction-adduction asymmetries.


Similar content being viewed by others
References
Parker JL, Santiago M. Oculomotor aspects of the hereditary cerebellar ataxias. Handb Clin Neurol. 2012;103:63–83.
Ohyagi Y, Yamada T, Okayama A, Sakae N, Yamasaki T, Ohshima T, et al. Vergence disorders in patients with spinocerebellar ataxia 3/Machado-Joseph disease: a synoptophore study. J Neurol Sci. 2000;173(2):120–3.
Ghasia FF, Wilmot G, Ahmed A, Shaikh AG. Strabismus and micro-opsoclonus in Machado-Joseph disease. Cerebellum. 2016;15(4):491–7.
von Noorden GK, Campos EC, editors. Binocular vision and ocular motility. 6th ed. St. Louis: Mosby; 2002. 672 p.
Luis L, Costa J, Muñoz E, de Carvalho M, Carmona S, Schneider E, et al. Vestibulo-ocular reflex dynamics with head-impulses discriminates spinocerebellar ataxias types 1, 2 and 3 and Friedreich ataxia. J Vestib Res. 2016;26(3):327–34.
Wu C, Chen D-B, Feng L, Zhou X-X, Zhang J-W, You H-J, et al. Oculomotor deficits in spinocerebellar ataxia type 3: potential biomarkers of preclinical detection and disease progression. CNS Neurosci Ther. 2017;23(4):321–8.
Furtado GV, De Oliveira CM, Bolzan G, Saute JAM, Saraiva-Pereira ML, Jardim LB. State biomarkers for Machado Joseph disease: validation, feasibility and responsiveness to change. Genet Mol Biol. 2019;42(1):238–51.
Versino M, Hurko O, Zee DS. Disorders of binocular control of eye movements in patients with cerebellar dysfunction. Brain. 1996;119(Pt 6):1933–50.
Lemos J, Novo A, Duque C, Castelhano J, Eggenberger E, Januário C. “Pinball” intrusions in spinocerebellar ataxia type 3. Neurology. 2018;90(1):36–7.
Shaikh AG, Ghasia FF. Misdirected horizontal saccades in pan-cerebellar atrophy. J Neurol Sci. 2015;355(1–2):125–30.
Rivaud-Pechoux S, Dürr A, Gaymard B, Cancel G, Ploner CJ, Agid Y, et al. Eye movement abnormalities correlate with genotype in autosomal dominant cerebellar ataxia type I. Ann Neurol. 1998;43(3):297–302.
Buttncr N, Geschwind D, Jen JC, Pcrlman S, Pulst SM, Baloh RW. Oculomotor phenotypes in autosomal dominant ataxias. Arch Neurol. 1998;55(10):1353–7.
Dawson DM, Feudo P, Zubick HH, Rosenberg R, Fowler H. Electro-oculographic findings in Machado-Joseph disease. Neurology. 1982;32(11):1272–6.
Schmitz-Hübsch T, Du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology. 2006;66(11):1717–20.
Hüfner K, Frenzel C, Kremmyda O, Adrion C, Bardins S, Glasauer S, et al. Esophoria or esotropia in adulthood: a sign of cerebellar dysfunction? J Neurol. 2015;262(3):585–92.
Rüb U, Gierga K, Brunt ER, De Vos RAI, Bauer M, Schöls L, et al. Spinocerebellar ataxias types 2 and 3: degeneration of the precerebellar nuclei isolates the three phylogenetically defined regions of the cerebellum. J Neural Transm. 2005;112(11):1523–45.
Rüb U, Bürk K, Schöls L, Brunt ER, De Vos RAI, Orozco Diaz G, et al. Damage to the reticulotegmental nucleus of the pons in spinocerebellar ataxia type 1, 2, and 3. Neurology. 2004;63(7):1258–63.
Tokumaru AM, Kamakura K, Maki T, Murayama S, Sakata I, Kaji T, et al. Magnetic resonance imaging findings of Machado-Joseph disease: histopathologic correlation. J Comput Assist Tomogr. 2003;27(2):241–8.
Takagi M, Tamargo R, Zee DS. Effects of lesions of the cerebellar oculomotor vermis on eye movements in primate: binocular control. Prog Brain Res. 2003;142:19–33.
Scherzed W, Brunt ER, Heinsen H, De Vos RA, Seidel K, Bürk K, et al. Pathoanatomy of cerebellar degeneration in spinocerebellar ataxia type 2 (SCA2) and type 3 (SCA3). Cerebellum. 2012;11(3):749–60.
Swartz BE, Li S, Bespalova I, Burmeister M, Dulaney E, Robinson FR, et al. Pathogenesis of clinical signs in recessive ataxia with saccadic intrusions. Ann Neurol. 2003;54(6):824–8.
Optican LM, Zee DS, Chu FC. Adaptive response to ocular muscle weakness in human pursuit and saccadic eye movements. J Neurophysiol. 1985;54(1):110–22.
Das VE. Strabismus and the oculomotor system: insights from macaque models. Annu Rev Vis Sci. 2016;2(1):37–59.
Acknowledgments
We would like to thank the patients, their relatives, and the neurology department staff for participating in the study.
Author information
Authors and Affiliations
Contributions
João Lemos: contributed to acquisition, analysis and interpretation of the data, drafting of the manuscript, study supervision, concept and design, and critical revision of manuscript for intellectual content; Ana Novo: contributed to acquisition, analysis and interpretation of the data, drafting of the manuscript, and critical revision of manuscript for intellectual content; Cristina Duque: contributed to acquisition and analysis and interpretation of the data; Inês Cunha: contributed to acquisition and analysis and interpretation of the data; Joana Ribeiro: contributed to acquisition and analysis and interpretation of the data; João Castelhano: contributed to acquisition and analysis and interpretation of the data; Cristina Januário: contributed to analysis and interpretation of the data, study concept and design, and critical revision of manuscript for intellectual content.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
João Lemos, MD, PhD and João Castelhano, MBE, PhD conducted the statistical analysis
Rights and permissions
About this article
Cite this article
Lemos, J., Novo, A., Duque, C. et al. Static and Dynamic Ocular Motor Abnormalities as Potential Biomarkers in Spinocerebellar Ataxia Type 3. Cerebellum 20, 402–409 (2021). https://doi.org/10.1007/s12311-020-01217-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-020-01217-4