Abstract
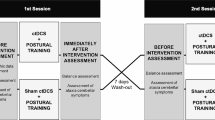
Cerebellar transcranial direct current stimulation (ctDCS) modulates cerebellar activity and postural control. However, the effects of ctDCS on postural control learning and the mechanisms associated with these effects remain unclear. To examine the effects of single-session ctDCS on postural control learning and cerebellar brain inhibition (CBI) of the primary motor cortex in healthy individuals. In this triple-blind, sham-controlled study, 36 participants were allocated randomly to one of three groups: (1) anodal ctDCS group, (2) cathodal ctDCS group, and (3) sham ctDCS group. ctDCS (2 mA) was applied to the cerebellar brain for 20 min prior to six blocks of standing postural control training (each block consisted of five trials of a 30-s tracking task). CBI and corticospinal excitability of the tibialis anterior muscle were assessed at baseline, immediately after, 1 day after, and 7 days after training. Skill acquisition following training was significantly reduced in both the anodal and cathodal ctDCS groups compared with the sham ctDCS group. Changes in performance measured 1 day after and 7 days after training did not differ among the groups. In the anodal ctDCS group, CBI significantly increased after training, whereas corticospinal excitability decreased. Anodal ctDCS-induced CBI changes were correlated with the learning formation of postural control (r = 0.55, P = 0.04). Single-session anodal and cathodal ctDCS could suppress the skill acquisition of postural control in healthy individuals. The CBI changes induced by anodal ctDCS may affect the learning process of postural control.







Similar content being viewed by others
References
Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527(3):633–9.
Ouchi Y, Okada H, Yoshikawa E, Nobezawa S, Futatsubashi M. Brain activation during maintenance of standing postures in humans. Brain. 1999;122(11):329–38.
Taube W, Mouthon M, Leukel C, Hoogewoud HM, Annoni JM, Keller M. Brain activity during observation and motor imagery of different balance tasks: an fMRI study. Cortex. 2015;64:102–14.
Ioffe ME, Chernikova LA, Ustinova KI. Role of cerebellum in learning postural tasks. Cerebellum. 2007;6:87–94.
Poortvliet P, Hsieh B, Cresswell A, Au J, Meinzer M. Cerebellar transcranial direct current stimulation improves adaptive postural control. Clin Neurophysiol. 2018;129:33–41.
Galea JM, Jayaram G, Ajagbe L, Celnik P. Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J Neurosci. 2009;29:9115–22.
Inukai Y, Saito K, Sasaki R, Kotan S, Nakagawa M, Onishi H. Influence of transcranial direct current stimulation to the cerebellum on standing posture control. Front Hum Neurosci. 2016;10:325.
Foerster Á, Melo L, Mello M, Castro R, Shirahige L, Rocha S, et al. Cerebellar transcranial direct current stimulation (ctDCS) impairs balance control in healthy individuals. Cerebellum. 2017;16:872–5.
Ehsani F, Samaei A, Zoghi M, Hedayati R, Jaberzadeh S. The effects of cerebellar transcranial direct current stimulation on static and dynamic postural stability in older individuals: a randomized double-blind sham-controlled study. Eur J Neurosci. 2017;46:2875–84.
Zandvliet SB, Meskers CGM, Kwakkel G, van Wegen EEH. Short-term effects of cerebellar tDCS on standing balance performance in patients with chronic stroke and healthy age-matched elderly. Cerebellum. 2018;17:575–89.
Steiner KM, Enders A, Thier W, Batsikadze G, Ludolph N, Ilg W, et al. Cerebellar tDCS does not improve learning in a complex whole body dynamic balance task in young healthy subjects. PLoS One. 2016;11:0163598.
Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I. Magnetic stimulation over the cerebellum in humans. Ann Neurol. 1995;37:703–13.
Fernandez L, Major BP, Teo WP, Byrne LK, Enticott PG. Assessing cerebellar brain inhibition (CBI) via transcranial magnetic stimulation (TMS): a systematic review. Neurosci Biobehav Rev. 2018;86:176–206.
Jayaram G, Galea JM, Bastian AJ, Celnik P. Human locomotor adaptive learning is proportional to depression of cerebellar excitability. Cereb Cortex. 2011;21:1901–9.
Jayaram G, Tang B, Pallegadda R, Vasudevan EV, Celnik P, Bastian A. Modulating locomotor adaptation with cerebellar stimulation. J Neurophysiol. 2012;107:2950–7.
Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat. 2005;4:287–91.
Ferrucci R, Cortese F, Priori A. Cerebellar tDCS: how to do it. Cerebellum. 2015;14:27–30.
Batsikadze G, Rezaee Z, Chang DI, Gerwig M, Herlitze S, Dutta A, et al. Effects of cerebellar transcranial direct current stimulation on cerebellar-brain inhibition in humans: a systematic evaluation. Brain Stimul. 2019;12:1177–86.
Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117:845–50.
Cacchio A, Cimini N, Alosi P, Santilli V, Marrelli A. Reliability of transcranial magnetic stimulation-related measurements of tibialis anterior muscle in healthy subjects. Clin Neurophysiol. 2009;120:414–9.
Kang N, Lee RD, Lee JH, Hwang MH. Functional balance and postural control improvements in patients with stroke after non-invasive brain stimulation: a meta-analysis. Arch Phys Med Rehabil. 2020;101(1):141–53.
Takakusaki K. Functional neuroanatomy for posture and gait control. J Mov Disord. 2017;10:1–17.
Dayan E, Cohen LG. Neuroplasticity subserving motor skill learning. Neuron. 2011;72(3):443–54.
Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol. 2016;127:1031–48.
Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M. Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul. 2009;2:201–7.
Benussi A, Dell'Era V, Cotelli MS, Turla M, Casali C, Padovani A, et al. Long term clinical and neurophysiological effects of cerebellar transcranial direct current stimulation in patients with neurodegenerative ataxia. Brain Stimul. 2017;10:242–50.
Winn P. How best to consider the structure and function of the pedunculopontine tegmental nucleus: evidence from animal studies. J Neurol Sci. 2006;248:234–50.
Matsugi A, Okada Y. Cerebellar transcranial direct current stimulation modulates the effect of cerebellar transcranial magnetic stimulation on the excitability of spinal reflex. Neurosci Res. 2020;150:37–43.
Takahashi Y, Fujiwara T, Yamaguchi T, Kawakami M, Mizuno K, Liu M. The effects of patterned electrical stimulation combined with voluntary contraction on spinal reciprocal inhibition in healthy individuals. Neuroreport. 2017;28(8):434–8.
Wessel MJ, Zimerman M, Timmermann JE, Heise KF, Gerloff C, Hummel FC. Enhancing consolidation of a new temporal motor skill by cerebellar noninvasive stimulation. Cereb Cortex. 2016;26:1660–7.
Herzfeld DJ, Pastor D, Haith AM, Rossetti Y, Shadmehr R, O'Shea J. Contributions of the cerebellum and the motor cortex to acquisition and retention of motor memories. Neuroimage. 2014;98:147–58.
Thirugnanasambandam N, Sparing R, Dafotakis M, Meister IG, Paulus W, Nitsche MA, et al. Isometric contraction interferes with transcranial direct current stimulation (tDCS) induced plasticity: evidence of state-dependent neuromodulation in human motor cortex. Restor Neurol Neurosci. 2011;29:311–20.
Ridding MC, Ziemann U. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J Physiol. 2010;588:2291–304.
Ni Z, Pinto AD, Lang AE, Chen R. Involvement of the cerebellothalamocortical pathway in Parkinson disease. Ann Neurol. 2010;68(6):816–24.
Schirinzi T, Di Lorenzo F, Ponzo V, Palmieri MG, Bentivoglio AR, Schillaci O, et al. Mild cerebello-thalamo-cortical impairment in patients with normal dopaminergic scans (SWEDD). Parkinsonism Relat Disord. 2016;28:23–8.
Carrillo F, Palomar FJ, Conde V, Diaz-Corrales FJ, Porcacchia P, Fernández-Del-Olmo M, et al. Study of cerebello-thalamocortical pathway by transcranial magnetic stimulation in Parkinson’s disease. Brain Stimul. 2013;6(4):582–9.
Schirinzi T, Di Lorenzo F, Sancesario GM, Di Lazzaro G, Ponzo V, Pisani A, et al. Amyloid-mediated cholinergic dysfunction in motor impairment related to Alzheimer’s disease. J Alzheimers Dis. 2018;64(2):525–32.
Di Lorenzo F, Bonnì S, Picazio S, Motta C, Caltagirone C, Martorana A, et al. Effects of cerebellar theta burst stimulation on contralateral motor cortex excitability in patients with Alzheimer’s disease. Brain Topogr. 2020;33(5):613–7.
Koch G, Bonnì S, Casula EP, Iosa M, Paolussi S, Pellicciari MC, et al. Effect of cerebellar stimulation on gait and balance recovery in patients with hemiparetic stroke: a randomized clinical trial. JAMA Neurol. 2019;76(2):170–8.
Koch G, Esposito R, Motta C, Casula EP, Di Lorenzo F, Bonnì S, et al. Improving visuo-motor learning with cerebellar theta burst stimulation: behavioral and neurophysiological evidence. Neuroimage. 2020;208:116424.
Mannarelli D, Pauletti C, Currà A, Marinelli L, Corrado A, Delle Chiaie R, et al. The cerebellum modulates attention network functioning: evidence from a cerebellar transcranial direct current stimulation and attention network test study. Cerebellum. 2019;18:457–68.
Yamaguchi T, Moriya K, Tanabe S, Kondo K, Otaka Y, Tanaka S. Transcranial direct-current stimulation combined with attention increases cortical excitability and improves motor learning in healthy volunteers. J Neuroeng Rehabil. 2020;17(1):23.
Kellermann T, Regenbogen C, De Vos M, Mößnang C, Finkelmeyer A, Habel U. Effective connectivity of the human cerebellum during visual attention. J Neurosci. 2012;32(33):11453–60.
Funding
Partial financial support was received from the Funds for a Grant-in-Aid for Young Scientists (18 K17723) to Tomofumi Yamaguchi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Research Involving Human Participants
This study was approved by the Ethics Review Board of Yamagata Prefectural University of Health Sciences (approval number: 1806-06) and was performed in accordance with the ethical standards established by the Declaration of Helsinki.
Informed Consent
All participants provided their written, informed consent before participating in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Katagiri, N., Kawakami, S., Okuyama, S. et al. Single-Session Cerebellar Transcranial Direct Current Stimulation Affects Postural Control Learning and Cerebellar Brain Inhibition in Healthy Individuals. Cerebellum 20, 203–211 (2021). https://doi.org/10.1007/s12311-020-01208-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-020-01208-5