Abstract
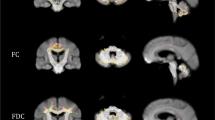
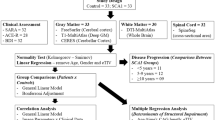
The spinocerebellar ataxias (SCAs) are a genetically heterogeneous group of cerebellar degenerative disorders, characterized by progressive gait unsteadiness, hand incoordination, and dysarthria. The mutational mechanism in SCA1, a dominantly inherited form of SCA, consists of an expanded trinucleotide CAG repeat. In SCA1, there is loss of Purkinje cells, neuronal loss in dentate nucleus, olives, and pontine nuclei. In the present study, we sought to apply intrinsic functional connectivity analysis combined with diffusion tensor imaging to define the state of cerebellar connectivity in SCA1. Our results on the intrinsic functional connectivity in lateral cerebellum and thalamus showed progressive organizational changes in SCA1 noted as a progressive increase in the absolute value of the correlation coefficients. In the lateral cerebellum, the anatomical organization of functional clusters seen as parasagittal bands in controls is lost, changing to a patchy appearance in SCA1. Lastly, only fractional anisotropy in the superior peduncle and changes in functional organization in thalamus showed a linear dependence to duration and severity of disease. The present pilot work represents an initial effort describing connectivity biomarkers of disease progression in SCA1. The functional changes detected with intrinsic functional analysis and diffusion tensor imaging suggest that disease progression can be analyzed as a disconnection syndrome.







Similar content being viewed by others
Notes
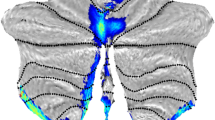
Anatomically [21], the lateral cerebellum included the following lobules: V lat (culmen inferior), VI lat (simplex), VIIA_Crus I/1,2 (superior semilunar lobule), VIIA_Crus II/1,2 (inferior semilunar lobule), VIIB lat (paramedian/gracilis), VIIIA lat (biventer, pars copularis), and VIIIB lat (biventer, pars paraflocculus dorsalis).
References
Koeppen AH. The hereditary ataxias. J Neuropathol Exp Neurol. 1998;57(6):531–43.
Klockgether T. Parkinsonism & related disorders. Ataxias. Parkinsonism Relat Disord. 2007;13 Suppl 3:S391–4.
Manto MU. The wide spectrum of spinocerebellar ataxias (SCAs). Cerebellum. 2005;4(1):2–6.
Mascalchi M. Spinocerebellar ataxias. Neurol Sci. 2008;29 Suppl 3:311–3.
Genis D, Matilla T, Volpini V, Rosell J, Davalos A, Ferrer I, et al. Clinical, neuropathologic, and genetic studies of a large spinocerebellar ataxia type 1 (SCA1) kindred: (CAG)n expansion and early premonitory signs and symptoms. Neurology. 1995;45(1):24–30.
Gilman S, Sima AA, Junck L, Kluin KJ, Koeppe RA, Lohman ME, et al. Spinocerebellar ataxia type 1 with multiple system degeneration and glial cytoplasmic inclusions. Ann Neurol. 1996;39(2):241–55.
Matilla-Duenas A, Goold R, Giunti P. Clinical, genetic, molecular, and pathophysiological insights into spinocerebellar ataxia type 1. Cerebellum. 2008;7(2):106–14.
Orr HT, Chung MY, Banfi S, Kwiatkowski Jr TJ, Servadio A, Beaudet AL, et al. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat Genet. 1993;4(3):221–6.
Schmitz-Hubsch T, Giunti P, Stephenson DA, Globas C, Baliko L, Sacca F, et al. SCA functional index: a useful compound performance measure for spinocerebellar ataxia. Neurology. 2008;71(7):486–92.
Lynch DR, Farmer JM, Tsou AY, Perlman S, Subramony SH, Gomez CM, et al. Measuring Friedreich ataxia: complementary features of examination and performance measures. Neurology. 2006;66(11):1711–6.
Lynch DR, Farmer JM, Wilson RL, Balcer LJ. Performance measures in Friedreich ataxia: potential utility as clinical outcome tools. Mov Disord. 2005;20(7):777–82.
Rabinovici GD, Roberson ED. Beyond diagnosis: what biomarkers are teaching us about the “bio”logy of Alzheimer disease. Ann Neurol. 2010;67(3):283–5.
Schmahmann JD, Pandya DN. Disconnection syndromes of basal ganglia, thalamus, and cerebrocerebellar systems. Cortex. 2008;44(8):1037–66.
Schmitz-Hubsch T, du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology. 2006;66(11):1717–20.
Noll DC, Cohen JD, Meyer CH, Schneider W. Spiral K-space MRI of cortical activation. J Magn Reson Imaging. 1995;5:49–56.
Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magn Reson Med. 1999;42(6):1014–8.
Habas C, Cabanis EA. Anatomical parcellation of the brainstem and cerebellar white matter: a preliminary probabilistic tractography study at 3 T. Neuroradiology. 2007;49(10):849–63.
Walsh RR, Small SL, Chen EE, Solodkin A. Network activation during bimanual movements in humans. Neuroimage. 2008;43:540–53.
Makris N, Schlerf JE, Hodge SM, Haselgrove C, Albaugh MD, Seidman LJ, et al. MRI-based surface-assisted parcellation of human cerebellar cortex: an anatomically specified method with estimate of reliability. Neuroimage. 2005;25(4):1146–60.
Schmahmann JD, Doyon J, Toga A, Petrides M, Evans A. MRI atlas of the human cerebellum. San Diego: Academic; 2000.
Makris N, Hodge SM, Haselgrove C, Kennedy DN, Dale A, Fischl B, et al. Human cerebellum: surface-assisted cortical parcellation and volumetry with magnetic resonance imaging. J Cogn Neurosci. 2003;15(4):584–99.
Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98(2):676–82.
Auer DP. Spontaneous low-frequency blood oxygenation level-dependent fluctuations and functional connectivity analysis of the ‘resting’ brain. Magn Reson Imaging. 2008;26(7):1055–64.
Diedrichsen J, Verstynen T, Schlerf J, Wiestler T. Advances in functional imaging of the human cerebellum. Curr Opin Neurol. 2010;23(4):382–7.
O’Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen-Berg H. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb Cortex. 2010;20(4):953–65.
Allen G, McColl R, Barnard H, Ringe WK, Fleckenstein J, Cullum CM. Magnetic resonance imaging of cerebellar-prefrontal and cerebellar-parietal functional connectivity. Neuroimage. 2005;28(1):39–48.
Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, et al. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 2009;29(26):8586–94.
Krienen FM, Buckner RL. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex. 2009;19(10):2485–97.
Baruchi I, Grossman D, Volman V, Hunter J, Towle VL, Ben-Jacob E. Functional holography analysis: simplifying the complexity of dynamical networks. In: Pecora L, Boccaletti S, editors. Stability of pattern formation in networks of dynamical systems. Chaos. 2006;16:15–112.
Baruchi I, Towle VL, Ben-Jacob E. Functional holography of complex networks activity—from cultures to the human brain. Complexity. 2005;10(3):38–51.
Baruchi I, Ben-Jacob E. Functional holography of recorded neuronal networks activity. Neuroinformatics. 2004;2(3):333–52.
Wolfram S. The mathematica book. 4th ed. Cambridge: Cambridge University Press; 1999.
MacQueen JB. Some methods for classification and analysis of multivariate observations. Proceedings of 5-th Berkeley Symposium on Mathematical Statistics and Probability. Berkley: University of California Press; 1967. p. 281–97
Grosberg AU. Statistical physics of macromolecules. New York: Springer; 1994.
Gavrilescu M, Stuart GW, Rossell S, Henshall K, McKay C, Sergejew AA, et al. Functional connectivity estimation in fMRI data: influence of preprocessing and time course selection. Hum Brain Mapp. 2008;29(9):1040–52.
Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA. 2006;103(37):13848–53.
Miller RA, Strominger NL. An experimental study of the efferent connections of the superior cerebellar peduncle in the rhesus monkey. Brain Res. 1977;133(2):237–50.
Prakash N, Hageman N, Hua X, Toga AW, Perlman SL, Salamon N. Patterns of fractional anisotropy changes in white matter of cerebellar peduncles distinguish spinocerebellar ataxia-1 from multiple system atrophy and other ataxia syndromes. Neuroimage. 2009;47 Suppl 2:T72–81.
Globas C, du Montcel ST, Baliko L, Boesch S, Depondt C, DiDonato S, et al. Early symptoms in spinocerebellar ataxia type 1, 2, 3, and 6. Mov Disord. 2008;23(15):2232–8.
Mandelli ML, De Simone T, Minati L, Bruzzone MG, Mariotti C, Fancellu R, et al. Diffusion tensor imaging of spinocerebellar ataxias types 1 and 2. AJNR Am J Neuroradiol. 2007;28(10):1996–2000.
Burk K, Abele M, Fetter M, Dichgans J, Skalej M, Laccone F, et al. Autosomal dominant cerebellar ataxia type I clinical features and MRI in families with SCA1, SCA2 and SCA3. Brain. 1996;119(Pt 5):1497–505.
Cummings CJ, Orr HT, Zoghbi HY. Progress in pathogenesis studies of spinocerebellar ataxia type 1. Philos Trans R Soc Lond B Biol Sci. 1999;354(1386):1079–81.
Ginestroni A, Dellanave R, Tessa C, Giannelli M, De Grandis D, Plasmati R, et al. Brain structural damage in spinocerebellar ataxia type 1: a VBM study. J Neurol. 2008;255(8):1153–8.
Klockgether T, Skalej M, Wedekind D, Luft AR, Welte D, Schulz JB, et al. Autosomal dominant cerebellar ataxia type I. MRI-based volumetry of posterior fossa structures and basal ganglia in spinocerebellar ataxia types 1, 2 and 3. Brain. 1998;121(Pt 9):1687–93.
Bowman AB, Lam YC, Jafar-Nejad P, Chen HK, Richman R, Samaco RC, et al. Duplication of Atxn1l suppresses SCA1 neuropathology by decreasing incorporation of polyglutamine-expanded ataxin-1 into native complexes. Nat Genet. 2007;39(3):373–9.
Burright EN, Clark HB, Servadio A, Matilla T, Feddersen RM, Yunis WS, et al. SCA1 transgenic mice: a model for neurodegeneration caused by an expanded CAG trinucleotide repeat. Cell. 1995;82(6):937–48.
Clark HB, Orr HT. Spinocerebellar ataxia type 1—modeling the pathogenesis of a polyglutamine neurodegenerative disorder in transgenic mice. J Neuropathol Exp Neurol. 2000;59(4):265–70.
Lam YC, Bowman AB, Jafar-Nejad P, Lim J, Richman R, Fryer JD, et al. ATAXIN-1 interacts with the repressor Capicua in its native complex to cause SCA1 neuropathology. Cell. 2006;127(7):1335–47.
Yamada M, Sato T, Tsuji S, Takahashi H. CAG repeat disorder models and human neuropathology: similarities and differences. Acta Neuropathol. 2008;115(1):71–86.
Giovannoni R, Maggio N, Rosaria Bianco M, Cavaliere C, Cirillo G, Lavitrano M, et al. Reactive astrocytosis and glial glutamate transporter clustering are early changes in a spinocerebellar ataxia type 1 transgenic mouse model. Neuron Glia Biol. 2007;3(4):335–51.
Lin X, Antalffy B, Kang D, Orr HT, Zoghbi HY. Polyglutamine expansion down-regulates specific neuronal genes before pathologic changes in SCA1. Nat Neurosci. 2000;3(2):157–63.
Serra HG, Byam CE, Lande JD, Tousey SK, Zoghbi HY, Orr HT. Gene profiling links SCA1 pathophysiology to glutamate signaling in Purkinje cells of transgenic mice. Hum Mol Genet. 2004;13(20):2535–43.
Brochu G, Maler L, Hawkes R. Zebrin II: a polypeptide antigen expressed selectively by Purkinje cells reveals compartments in rat and fish cerebellum. J Comp Neurol. 1990;291(4):538–52.
Hawkes R, Herrup K. Aldolase C/zebrin II and the regionalization of the cerebellum. J Mol Neurosci. 1995;6(3):147–58.
Leclerc N, Dore L, Parent A, Hawkes R. The compartmentalization of the monkey and rat cerebellar cortex: zebrin I and cytochrome oxidase. Brain Res. 1990;506(1):70–8.
Leclerc N, Schwarting GA, Herrup K, Hawkes R, Yamamoto M. Compartmentation in mammalian cerebellum: zebrin II and P-path antibodies define three classes of sagittally organized bands of Purkinje cells. Proc Natl Acad Sci USA. 1992;89(11):5006–10.
Pakan JM, Iwaniuk AN, Wylie DR, Hawkes R, Marzban H. Purkinje cell compartmentation as revealed by zebrin II expression in the cerebellar cortex of pigeons (Columba livia). J Comp Neurol. 2007;501(4):619–30.
Sillitoe RV, Kunzle H, Hawkes R. Zebrin II compartmentation of the cerebellum in a basal insectivore, the Madagascan hedgehog tenrec Echinops telfairi. J Anat. 2003;203(3):283–96.
Sillitoe RV, Malz CR, Rockland K, Hawkes R. Antigenic compartmentation of the primate and tree shrew cerebellum: a common topography of zebrin II in Macaca mulatta and Tupaia belangeri. J Anat. 2004;204(4):257–69.
Buono P, D’Armiento FP, Terzi G, Alfieri A, Salvatore F. Differential distribution of aldolase A and C in the human central nervous system. J Neurocytol. 2001;30(12):957–65.
Armstrong CL, Hawkes R. Pattern formation in the cerebellar cortex. Biochem Cell Biol. 2000;78(5):551–62.
Pijpers A, Voogd J, Ruigrok TJ. Topography of olivo-cortico-nuclear modules in the intermediate cerebellum of the rat. J Comp Neurol. 2005;492(2):193–213.
Sugihara I, Quy PN. Identification of aldolase C compartments in the mouse cerebellar cortex by olivocerebellar labeling. J Comp Neurol. 2007;500(6):1076–92.
Sugihara I, Shinoda Y. Molecular, topographic, and functional organization of the cerebellar cortex: a study with combined aldolase C and olivocerebellar labeling. J Neurosci. 2004;24(40):8771–85.
Eccles J, Llinas R, Sasaki K. Excitation of cerebellar Purkinje cells by the climbing fibres. Nature. 1964;203:245–6.
Kazantsev VB, Nekorkin VI, Makarenko VI, Llinas R. Olivo-cerebellar cluster-based universal control system. Proc Natl Acad Sci USA. 2003;100(22):13064–8.
Llinas R, Leznik E, Makarenko VI. On the amazing olivocerebellar system. Ann NY Acad Sci. 2002;978:258–72.
Llinas R, Nicholson C. Reversal properties of climbing fiber potential in cat Purkinje cells: an example of a distributed synapse. J Neurophysiol. 1976;39(2):311–23.
Llinas RR. Inferior olive oscillation as the temporal basis for motricity and oscillatory reset as the basis for motor error correction. Neuroscience. 2009;162(3):797–804.
Sugihara I, Lang EJ, Llinas R. Serotonin modulation of inferior olivary oscillations and synchronicity: a multiple-electrode study in the rat cerebellum. Eur J Neurosci. 1995;7(4):521–34.
Welsh JP, Lang EJ, Suglhara I, Llinas R. Dynamic organization of motor control within the olivocerebellar system. Nature. 1995;374(6521):453–7.
Yamamoto T, Fukuda M, Llinas R. Bilaterally synchronous complex spike Purkinje cell activity in the mammalian cerebellum. Eur J Neurosci. 2001;13(2):327–39.
Sugihara I, Lang EJ, Llinas R. Uniform olivocerebellar conduction time underlies Purkinje cell complex spike synchronicity in the rat cerebellum. J Physiol. 1993;470:243–71.
Welsh JP, Llinas R. Some organizing principles for the control of movement based on olivocerebellar physiology. Prog Brain Res. 1997;114:449–61.
Sugihara I, Marshall SP, Lang EJ. Relationship of complex spike synchrony bands and climbing fiber projection determined by reference to aldolase C compartments in crus IIa of the rat cerebellar cortex. J Comp Neurol. 2007;501(1):13–29.
Lang EJ. Organization of olivocerebellar activity in the absence of excitatory glutamatergic input. J Neurosci. 2001;21(5):1663–75.
Lang EJ. GABAergic and glutamatergic modulation of spontaneous and motor-cortex-evoked complex spike activity. J Neurophysiol. 2002;87(4):1993–2008.
Blenkinsop TA, Lang EJ. Block of inferior olive gap junctional coupling decreases Purkinje cell complex spike synchrony and rhythmicity. J Neurosci. 2006;26(6):1739–48.
Marshall SP, Lang EJ. Inferior olive oscillations gate transmission of motor cortical activity to the cerebellum. J Neurosci. 2004;24(50):11356–67.
Serra HG, Duvick L, Zu T, Carlson K, Stevens S, Jorgensen N, et al. RORalpha-mediated Purkinje cell development determines disease severity in adult SCA1 mice. Cell. 2006;127(4):697–708.
Duenas AM, Goold R, Giunti P. Molecular pathogenesis of spinocerebellar ataxias. Brain. 2006;129(Pt 6):1357–70.
Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004;10(Suppl):S10–7.
Skinner PJ, Vierra-Green CA, Clark HB, Zoghbi HY, Orr HT. Altered trafficking of membrane proteins in Purkinje cells of SCA1 transgenic mice. Am J Pathol. 2001;159(3):905–13.
Lang EJ, Sugihara I, Llinas R. GABAergic modulation of complex spike activity by the cerebellar nucleoolivary pathway in rat. J Neurophysiol. 1996;76(1):255–75.
Della Nave R, Ginestroni A, Tessa C, Salvatore E, De Grandis D, Plasmati R, et al. Brain white matter damage in SCA1 and SCA2. An in vivo study using voxel-based morphometry, histogram analysis of mean diffusivity and tract-based spatial statistics. Neuroimage. 2008;43(1):10–9.
Guerrini L, Lolli F, Ginestroni A, Belli G, Della Nave R, Tessa C, et al. Brainstem neurodegeneration correlates with clinical dysfunction in SCA1 but not in SCA2. A quantitative volumetric, diffusion and proton spectroscopy MR study. Brain. 2004;127(Pt 8):1785–95.
Akkal D, Dum RP, Strick PL. Supplementary motor area and presupplementary motor area: targets of basal ganglia and cerebellar output. J Neurosci. 2007;27(40):10659–73.
Dum RP, Strick PL. An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. J Neurophysiol. 2003;89(1):634–9.
Hoover JE, Strick PL. The organization of cerebellar and basal ganglia outputs to primary motor cortex as revealed by retrograde transneuronal transport of herpes simplex virus type 1. J Neurosci. 1999;19(4):1446–63.
Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003;23(23):8432–44.
Middleton FA, Strick PL. Cerebellar output channels. Int Rev Neurobiol. 1997;41:61–82.
Middleton FA, Strick PL. Dentate output channels: motor and cognitive components. Prog Brain Res. 1997;114:553–66.
Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16(3):367–78.
Schmahmann JD, Caplan D. Cognition, emotion and the cerebellum. Brain. 2006;129(Pt 2):290–2.
Della Nave R, Foresti S, Tessa C, Moretti M, Ginestroni A, Gavazzi C, et al. ADC mapping of neurodegeneration in the brainstem and cerebellum of patients with progressive ataxias. Neuroimage. 2004;22(2):698–705.
Acknowledgments
We want to thank Mr. R. Lyons for technical help in performing the scan sessions, Ms. N. Sansone for assisting in the generation of anatomical ROIs, and Ms. N. Lobo for assisting in primary MRI analysis. Special thanks go to Dr. Christian Hansel for his valuable comments. The work was supported by grants from the Center for Integrative Neuroscience and Neuroengineering Research (CINNR), NIH RO1-NS-54942, and the James McDonnell Foundation (NRG group).
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Solodkin, A., Peri, E., Chen, E.E. et al. Loss of Intrinsic Organization of Cerebellar Networks in Spinocerebellar Ataxia Type 1: Correlates with Disease Severity and Duration. Cerebellum 10, 218–232 (2011). https://doi.org/10.1007/s12311-010-0214-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-010-0214-5