Abstract
Background
B-lymphoblastic leukemia/lymphomas (B-ALL/LBL) are uncommon neoplasms that may be associated with a variety of cytogenetic and molecular changes. The mechanisms by which these changes arise have not been fully described.
Aims/Purpose
This report describes an unusual case of B-ALL/LBL with complex clonal evolution that includes BCL2 and MYC gene rearrangements.
Methods
Immunophenotyping was performed by immunohistochemistry and flow cytometry. Traditional G-band karyotyping was accompanied by fluorescence in-situ hybridization (FISH) using break-apart and dual fusion probes. Single nucleotide polymorphisms were assessed using a high-density DNA microarray.
Results
The karyotype of the blasts showed reciprocal translocation of chromosomes 4 and 18, reciprocal translocation of chromosomes 8 and 14 with two copies of the oncogenic translocation derivative(14)t(8;14), and no normal chromosome 14. FISH studies showed complex IGH-BCL2 and IGH-MYC fusion signals.
Conclusions
A clonal evolution model involving multiple chromosomal translocations and mitotic recombination is postulated to account for the karyotype, FISH, and microarray results but leaves unresolved the exact order of the evolutionary changes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aggressive hematologic malignancies sometimes harbor multiple structural and/or numerical chromosomal abnormalities detected by cytogenetics, FISH, or microarray techniques. These abnormalities are thought to arise from clonal evolution in which accumulation of genetic changes confers growth advantage to subclones of the original tumor leading to tumor progression [1]. Hematolymphoid neoplasms often show evidence of clonal evolution in their karyotypes and/or gene mutational profiles either at diagnosis or relapse after chemotherapy [2,3,4]. This report describes an unusual case of B-ALL/LBL with complex MYC and BCL2 gene rearrangements. A step-wise process for clonal evolution of the disease is proposed based on cytogenetic, FISH, and microarray studies.
Clinical history
An 83-year-old man presented with complaints of generalized weakness, dizziness, and 30-pound weight loss over 2 months. His medical history was negative for leukemia or lymphoma. Computerized axial tomography of the chest, abdomen, and pelvis showed neither lymphadenopathy nor hepatosplenomegaly. Laboratory analysis showed hemoglobin 13.5 g/dl, platelet count 36,000/µl, and white blood cell count 11,900/µl with 56% neutrophils, 15% bands, 5% myelocytes, 1% monocytes, 10% lymphocytes, and 13% blasts. After diagnosis, the patient declined treatment and transitioned to hospice care.
Materials and methods
Immunohistochemistry
Immunohistochemical staining was performed on a Ventana Benchmark Ultra system using validated automated operating protocols. Ventana monoclonal antibodies used were CD20 (L26), PAX-5 (SP34), CD3 (2GV6), BCL-2 (124), MYC (Y69), and Ki-67 (SP6). Cell Marque monoclonal antibodies used were CD10 (56C6) and BCL6 (G191E/A8).
Flow cytometry
Flow cytometry of the bone marrow aspirate was performed by Accupath Diagnostic Laboratories (Brentwood, TN, USA) using 8-color flow analysis performed on a Becton–Dickinson (BD) FACSLyric™ flow cytometer.
Karyotype and FISH
Chromosome analysis and FISH were performed on the same bone marrow aspirate by a reference laboratory (Integrated Oncology, RTP, NC, USA). FISH was performed using break-apart probes for MYC, BCL2, BCL6, PDGFRA and dual fusion probes targeting IGH-MYC and IGH-BCL2 (Kreatech, Leica Biosystems). Breakapart FISH probes were used in the initial screening for gene rearrangements. Dual fusion FISH probes were used to characterize specific gene rearrangements.
DNA microarray
The Cytoscan HD chip and GeneChip instrument system (Affymetrix, Thermo Fisher Scientific) was used for whole genome single nucleotide polymorphism (SNP) microarray analysis. Propriety software (CHAS) from Applied Biosystems was utilized for chromosome analysis of microarray data to determine SNP genotypes based on the GRCh37/hg19 assembly.
Results
Bone marrow biopsy and immunophenotyping
Bone marrow aspirate smears showed approximately 70% blasts. Blasts were variable in size with immature chromatin, indistinct nucleoli, deep blue cytoplasm and prominent cytoplasmic vacuoles (Fig. 1a). A few mitotic figures were noted. A hypercellular bone marrow core biopsy showed leukemic infiltration by blasts (Fig. 1b and c) corresponding to those in the aspirate smear. Some residual hematopoiesis was present. Selected immunohistochemical stains are shown in Fig. 1d–i. Blasts were positive by immunohistochemistry for PAX5, CD10, BCL2, MYC, and BCL6 (subset) with approximately 90% of the blasts positive for Ki-67. Blasts were negative for CD20, CD3, and EBER-ISH. Flow cytometry of the peripheral blood and bone marrow showed a population of B cells that expressed CD45(dim), HLA-DR, CD19, CD10, CD22, cCD22, cCD79a(+ / −), and CD38. The B cells were negative for CD20, CD34, TdT, sIg, cIg, cCD3, MPO, CD13, CD33, CD117, CD11b, CD11c, CD14, CD64, CD15, CD56, CD2, CD3, CD5, and CD7.
Bone marrow aspirate and bone marrow core biopsy with immunohistochemical stains: (a) Bone marrow aspirate, Wright-Geimsa stain, 1000 × magnification, (b) Core biopsy, hematoxylin and eosin stain, 200 × magnification, (c) Core biopsy, hematoxylin and eosin stain, 600 × magnification, (d–i) Immunohistochemical stains of the core biopsy with markers indicated within each panel
Karyotype and FISH
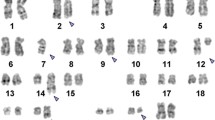
Cytogenetic analysis of GTG banded metaphases from B-mitogen stimulated cultures of the bone marrow aspirate (Fig. 2) showed 47,XY,t(4;18)(q12;q23), + 7,t(8;14)(q24;q32),der(14)t(8;14)(q24;q32) [20]. The karyotype of the abnormal lymphoid clone was characterized by a reciprocal translocation involving chromosomes 4 and 18 and a reciprocal translocation involving chromosomes 8 and 14. In addition, all cells contained an extra translocation derivative 14 of the t(8;14) in place of the normal chromosome 14 as well as an extra copy of chromosome 7. Interphase FISH of bone marrow aspirate showed that 92% of the cells were positive for three MYC-IGH fusion signals (Fig. 3a). Interphase FISH also showed that 89% of the cells were positive for two BCL2-IGH fusion signals (Fig. 3b). FISH was negative for BCL6 and PDGFRA rearrangement. A more detailed analysis was performed by constructing MYC-IGH and BCL2-IGH metaphase FISH karyotypes (Fig. 3c and d). In Fig. 3c, the MYC-IGH fusions (yellow) are present on two identical copies of the derivative 14 and the translocation derivative 8. In addition, a MYC signal (red) is present on the normal 8 and an IGH signal (green) is seen on the translocation derivative 4. In Fig. 3d, the BCL2-IGH fusions (yellow) are present on the 4 and 8 translocation derivatives. A BCL2 signal (red) is present on the normal 18 and IGH signals (green) are present on the derivative 14 chromosomes.
FISH of the bone marrow aspirate: (a) Interphase FISH with dual fusion MYC (red), IGH (green), and centromere 8 (blue) probes, (b) Interphase FISH with dual fusion BCL2 (red) and IGH (green) probes, (c) Metaphase FISH karyotype with dual fusion MYC (red), IGH (green), and centromere 8 (blue) probes, (d) Metaphase FISH karyotype with dual fusion BCL2 (red) and IGH (green) probes
SNP microarray
Whole genome SNP microarray analysis showed allele heterozygosity of chromosome 14 from the centromere to the immunoglobin heavy chain locus near the long arm terminus. In addition, the array demonstrated a gain of all of chromosome 7 (detected also by karyotype), a gain of distal 8q from the MYC gene at q24.21 to the telomere, and three microdeletions in chromosome 9. The latter included an approximately 0.5 Mb single copy loss at 9p13.2 spanning the PAX5 gene and bi-allelic deletions of different sizes at 9p21.3 spanning the CDKN2A tumor suppressor. The SNP microarray also showed an extended contiguous region of allele homozygosity in the p terminus of chromosome 6 consistent with acquired copy-neutral loss of heterozygosity (CN-LOH) (data not shown).
Discussion
Precursor B-cell neoplasms with IGH-MYC rearrangement have been well documented [5, 6]. The leukemic B-cell neoplasm described in this case report, however, has unique features that make it difficult to classify. The clinical presentation, precursor B-cell immunophenotype (CD45(dim), CD19, PAX5, CD10, CD22, cCD22, and cCD79a), negative CD20, and karyotype/FISH results are consistent with a diagnosis of either “B-ALL with MYC rearrangement” in the 2022 ICC classification [7] or “B-ALL/LBL with other defined genetic abnormalities” in the 2022 WHO classification [8]. The absence of immunoglobulin expression and deletion of CDKN2A, although not specific for B-ALL/LBL, lend support to the diagnosis. However, the negative TdT and expression of BCL6 in a subset of malignant cells raise the possibility of a high grade B-cell lymphoma with MYC and BCL2 rearrangements [9] or a lymphoblastic transformation of follicular lymphoma [10], although there is no clinical evidence to support these diagnoses.
The complex karyotype and FISH results shown in Figs. 2 and 3 are strongly suggestive of multistep leukemogenesis driven by clonal evolution. Postulated intermediate steps in the clonal evolution process are illustrated in Fig. 4. The first step is proposed to be a t(14;18)(q32;q21) reciprocal translocation that results in the juxtaposition of the BCL2 and IGH loci with the oncogenic driver on the der(14)t(14;18) (Fig. 4b). The second step is postulated to be t(8;14)(q24;q32) reciprocal translocation which causes juxtaposition of the MYC and IGH loci with the oncogenic driver on the der(14)t(8;14) (Fig. 4c). The next steps are proposed to be der(14) mitotic recombination (MR) and t(4;18)(q12;q23) reciprocal translocation. The MR duplicates the MYC-IGH fusion from the der(14)t(8;14) to its homologous chromosome (Fig. 4d). It is postulated from the targeted FISH studies that this MR displaced the oncogenic BCL2-IGH fusion from the der(14)t(14;18) to the der(8)t(8;14) distal to the non-oncogenic MYC-IGH fusion (see dashed arrows in Fig. 4c) to create the G-band cryptic der(8)t(8;14;18). The t(4;18)(q12;q23) presumptively involved an original der(18)t(14;18) and a normal copy of chromosome 4 to create a cryptic der(4)t(4;14;18) and der(18)t(4;18) (Fig. 4d). Because it is not possible to determine the order of the t(4;18) and der(14) MR events, these two steps in the evolution of the neoplastic clone are illustrated as occurring simultaneously in Fig. 4d.
Stepwise model for clonal evolution of the patient’s B-ALL involves reciprocal translocations and mitotic recombination. Oncogenic translocation derivatives are indicated in bold type: (a) Normal chromosome pairs 8, 14, 18 and 4 with chromosomal locations of MYC (8q24), IGH (14q32), and BCL2 (18q21) indicated, (b) Reciprocal translocation of chromosomes 14 and 18, (c) Reciprocal translocation of chromosomes 8 and 14, (d) Mitotic recombination involving the two translocation derivative 14 chromosomes with displacement of the BCL2-IGH fusion from the der(14)t(14;18) to the translocation derivative 8 chromosome as indicated by dashed arrows in 4c; reciprocal translocation of chromosome 4 and der(18)t(14;18)
Although the proposed model of clonal evolution in Fig. 4 accounts for the karyotype and FISH data, it is not possible to determine with certainty the sequence of events because intermediate clones in the evolution of the patient’s leukemia were not extant at clinical presentation of the disease and no subclones were detected by cytogenetics. It is logical, however, to start the clonal evolution sequence with a low-grade t(14;18) translocation followed by a high-grade t(8;14) translocation and duplication of the der(14)t(8;14) chromosome. In this context, it is unclear how the t(4;18)(q12;q23) translocation may lead to a selective growth advantage of the leukemic clone. One mechanism may be activation of an oncogene at the translocation breakpoint. To investigate this possibility, PDGFRA at 4q12 was tested and ruled out by FISH. However, activation of an unknown oncogene at 4q12 or 18q23 may be postulated. Alternatively, the t(4;18) translocation may represent a sporadic event that does not confer a growth advantage to the clone.
The B-ALL/LBL case in this report is unique because the BCL2 rearrangement was detected by FISH without the t(14;18) appearing in the karyotype, secondary to separate rearrangements of both translocation derivatives. The novel retention of the oncogenic BCL2-IGH fusion, apparently moved from the der(14)t(14;18) to the der(8)t(8;14;18), is also a unique feature of this case because nearly all MR leads to loss of the displaced segment. The proposed model provides insight into possible mechanisms of clonal evolution in leukemia and other hematologic malignancies.
References
Nowell PC (1976) The clonal evolution of tumor cell populations. Science 194:23–28. https://doi.org/10.1126/science.959840
Jan M, Majeti R (2013) Clonal evolution of acute leukemia genomes. Oncogene 32:135–40. https://doi.org/10.1038/onc.2012.48
Ferrando A, López-Otín C (2017) Clonal evolution in leukemia. Nat Med 23:1135–1145. https://doi.org/10.1038/nm.4410
Reutter K, Sandmann S, Rohde J et al (2021) Reconstructing clonal evolution in relapsed and non-relapsed Burkitt lymphoma. Leukemia 35:639–643. https://doi.org/10.1038/s41375-020-0862-5
Li Y, Gupta G, Molofsky A, Xie Y, Shihabi N, McCormick J, Jaffe ES (2018) B Lymphoblastic leukemia/lymphoma with Burkitt-like morphology and IGH/MYC rearrangement: report of 3 cases in adult patients. Am J Surg Pathol 42:269–276. https://doi.org/10.1097/PAS.0000000000000982
Wagener R, López C, Kleinheinz K et al (2018) IG-MYC+ neoplasms with precursor B-cell phenotype are molecularly distinct from Burkitt lymphomas. Blood 132:2280–2285. https://doi.org/10.1182/blood-2018-03-842088
Arber DA, Orazi A, Hasserjian RP et al (2022) International consensus classification of myeloid neoplasms and acute leukemias: integrating morphologic, clinical, and genomic data. Blood 140:1200–1228. https://doi.org/10.1182/blood.2022015850
Alaggio R, Amador C, Anagnostopoulos I, et al (2022) The 5th edition of the World Health Organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia. 36:1720–1748. https://doi.org/10.1038/s41375-022-01620-2
Moench L, Sachs Z, Aasen G, Dolan M, Dayton V, Courville EL (2016) Double- and triple-hit lymphomas can present with features suggestive of immaturity, including TdT expression, and create diagnostic challenges. Leuk Lymphoma 57:2626–35. https://doi.org/10.3109/10428194.2016.1143939
Slot LM, Hoogeboom R, Smit LA et al (2016) B-lymphoblastic lymphomas evolving from follicular lymphomas co-express surrogate light chains and mutated gamma heavy chains. Am J Pathol 186:3273–3284. https://doi.org/10.1016/j.ajpath.2016.07.027
Acknowledgements
The authors thank Kim Hall and Dr. Krishna Oza for providing methods of immunohistochemistry and flow cytometry. Dr. David Viswanatha and Dr. Timothy O’Leary provided helpful comments and suggestions for improving the manuscript.
Funding
This study was not supported by any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The Central Arkansas Veterans Healthcare System IRB has determined that this case report is not research.
Informed consent
For this type of study, formal consent is not required. For this type of study, informed consent is not required.
Consent for publication
For this type of study, consent for publication is not required.
Conflict of Interest
The authors declare no competing interests or conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schichman, S.A., Penton, A.L., Ghanta, S.N. et al. B-lymphoblastic leukemia/lymphoma with MYC and BCL2 gene rearrangements shows evidence for clonal evolution and mitotic recombination. J Hematopathol 16, 111–117 (2023). https://doi.org/10.1007/s12308-023-00541-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12308-023-00541-y