Abstract
Multiple malignancies may occur in the same patient, and a few reports describe cases with multiple hematologic and non-hematologic neoplasms. We report the case of a patient who showed the sequential occurrence of four different lymphoid neoplasms together with a squamous cell carcinoma of the lung. A 62-year-old man with adenopathy was admitted to the hospital, and lymph node biopsy was positive for low-grade follicular lymphoma. He achieved a partial remission with chemotherapy. Two years later, a PET-CT scan showed a left hilar mass in the lung; biopsy showed a squamous cell carcinoma. Simultaneously, he was diagnosed with diffuse large B cell lymphoma in a neck lymph node; after chemo- and radiotherapy, he achieved a complete response. A restaging PET-CT scan 2 years later revealed a retroperitoneal nodule, and biopsy again showed a low-grade follicular lymphoma, while a biopsy of a cutaneous scalp lesion showed a CD30-positive peripheral T cell lymphoma. After some months, a liver biopsy and a right cervical lymph node biopsy showed a CD30-positive peripheral T cell lymphoma consistent with anaplastic lymphoma kinase-negative anaplastic large cell lymphoma. Flow cytometry and cytogenetic and molecular genetic analysis performed at diagnosis and during the patient’s follow-up confirmed the presence of two clonally distinct B cell lymphomas, while the two T cell neoplasms were confirmed to be clonally related. We discuss the relationship between multiple neoplasms occurring in the same patient and the various possible risk factors involved in their development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The appearance of multiple malignancies in the same patient is a rare occurrence, which can either be synchronous or metachronous. The incidence of multiple malignancies varies with age, sex, geographic origin, and site and type of tumors. The etiology is multifactorial, and some of the factors involved in the pathogenesis of these conditions include genetic predisposition, immunodeficiency, radiation therapy, chemotherapy, and various infectious agents, including Epstein–Barr virus (EBV) [1]. There are several reports of patients with a hematologic malignancy who develop a non-hematologic cancer [2–5], but only few case reports describe patients with multiple hematologic and non-hematologic malignancies [6, 7]. We describe here the unusual case of a patient who developed low-grade follicular lymphoma, squamous cell carcinoma of the lung, diffuse large B cell lymphoma (DLBCL) with a clonality distinct from the follicular lymphoma, peripheral T cell lymphoma of the skin, and systemic peripheral T cell lymphoma consistent with anaplastic lymphoma kinase (ALK)-negative anaplastic large cell lymphoma (ALCL) that was clonally related to the cutaneous lymphoma. We discuss the possibility of a relationship between these different neoplasms developing in the same patient and the importance of understanding various risk factors involved in the development of multiple malignancies.
Case history
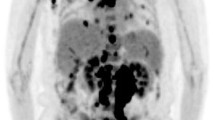
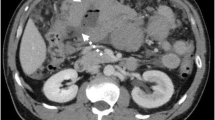
In 2003, a 62-year-old former smoker with known asbestos exposure was admitted to the hospital for chest pain and cough. He reported a family history of malignancies, in that his father died of lip cancer, a maternal uncle from lymphoma and lung cancer, and a second cousin from lung cancer. Chest X-ray demonstrated adenopathy, and a paratracheal lymph node biopsy showed grade 1 of 3 follicular lymphoma (Table 1). Conventional cytogenetics performed on the lymph node revealed a complex karyotype, with a t(14;18); flow cytometry revealed a lambda light-chain-restricted B cell population (Table 2, Fig. 1a). Staging PET scan showed lymphoma involving the left chest, neck, and spleen. He was treated with three cycles of fludarabine, Cytoxan and Rituxan (FCR). The patient had a partial remission and was followed up every 4 months. In late 2005, he presented to his oncologist after an episode of hemoptysis. A chest X-ray showed a left hilar lesion. A subsequent PET-CT scan showed a left hilar mass, a 9-cm mass in the spleen, and additional PET avid lymph nodes in the left neck, paraesophageal region, and abdomen. The patient then had a bronchoscopy and transbronchial biopsy; the biopsy showed a poorly differentiated squamous cell carcinoma (Table 1). In early 2006, a left neck lymph node was excised on which a diagnosis of DLBCL was rendered (Table 1, Fig. 2a–c). Cytogenetic analysis of the lymph node revealed a BCL6 gene rearrangement (confirmed by metaphase fluorescence in situ hybridization [FISH]) and a complex karyotype distinct from the prior findings of 2003, and flow cytometric analysis revealed a kappa light-chain-restricted B cell population (Table 2, Fig. 1b). Immunoglobulin heavy chain (IgH) gene rearrangement studies by polymerase chain reaction (PCR) showed the presence of a clonal B cell population (Table 2, Fig. 4b–d). Since the patient was not a surgical candidate for the non-small cell lung cancer, two cycles of cisplatin and etoposide (VP-16) were administered. In order to maximize the therapeutic effect on the DLBCL, rituximab was added to the regimen. Following this initial induction chemotherapy, eight cycles of rituximab, ifosfamide, carboplatin, and etoposide and radiotherapy were administered. The treatment course was complicated by expected myelosuppression, but he achieved a complete response. In March 2008, a restaging total body PET-CT scan revealed new left retroperitoneal nodules and enlarging right internal iliac chain lymph nodes suspicious for lymphoma recurrence. A retroperitoneal nodule was biopsied and found to contain an atypical lymphoid proliferation consistent with grade 1 of 3 follicular lymphoma (Table 1, Fig. 2d–f). Interestingly, flow cytometric analysis of this lymphoma revealed a clonal lambda light chain population, similar to that seen in 2003, while the analysis of 2006 performed on the DLBCL revealed a clonal kappa light chain population (Table 2). IgH gene rearrangement studies of the 2008 follicular lymphoma showed a clonal rearrangement with a different-sized PCR product from the 2006 DLBCL (Table 2, Fig. 4a–d). Finally, FISH analysis of paraffin-embedded tissue from the 2008 follicular lymphoma demonstrated the presence of a BCL2 gene rearrangement and absence of a BCL6 gene rearrangement (Table 2). The patient was treated with four doses of rituximab administered weekly. Just prior to his follicular lymphoma recurrence, in February 2008, the patient developed a scalp lesion that was biopsied and showed a diffuse CD30-positive, ALK-negative cutaneous T cell infiltrate (Table 1, Fig. 3d–f). The lesion spontaneously resolved without further treatment, and a primary cutaneous CD30-positive T cell lymphoproliferative disorder was the presumed diagnosis. However, in October 2008, a restaging total body CT scan revealed progressive adenopathy and enlarging liver and spleen lesions. Biopsies of the liver and right cervical lymph node showed a peripheral T cell lymphoma consistent with ALK-negative ALCL (Table 1, Fig. 3a–c). T cell receptor (TCR) gene rearrangement studies of the right cervical lymph node and scalp biopsies showed clonal T cell populations with PCR products of similar size, confirming that the two T cell lymphomas were clonally related and supporting a diagnosis of systemic ALK-negative ALCL with initial manifestation in the skin (Table 2). The patient was treated with six cycles of dose-reduced cyclophosphamide, doxorubicin, vincristine, and prednisone and recently underwent non-myeloablative allogeneic stem cell transplant from a matched-related donor.
Cytogenetic analysis of the patient's B cell lymphomas. Cytogenetic analysis of the 2003 lymph node showing grade 1 follicular lymphoma (a) revealed a complex karyotype (arrows), with a translocation between chromosomes 14 and 18 indicating a rearrangement involving IGH and BCL2. Analysis of the 2006 lymph node with DLBCL also showed a complex karyotype (arrows), which was distinct from that of the prior follicular lymphoma (b). In particular, t(14;18) was absent. Metaphase FISH analysis revealed a BCL6 rearrangement involving chromosome 3 (not shown). For complete karyotypes, see Table 2
Histologic and immunohistochemical findings of the patient's B cell lymphomas. Biopsy of enlarged left neck lymph node from 2006 revealed an infiltrate of large atypical lymphoid cells with oval, irregular, and multilobated nuclei and prominent nucleoli (a). The cells effaced the nodal architecture in a diffuse pattern (b) and were positive for Pax5 (c), consistent with DLBCL. In 2008, a retroperitoneal lymph node core biopsy showed a proliferation of small lymphocytes with irregular nuclei, consistent with centrocytes (d), forming vague follicle structures (e) and staining positively for Pax5 (f). The findings were consistent with relapsed follicular lymphoma, grade 1 of 3
Histologic and immunohistochemical findings of the patient’s T cell lymphomas. In 2008, biopsy of an enlarged right neck lymph node showed a diffuse proliferation of large atypical lymphoid cells with round to irregular nuclei, sometimes reniform nuclei; mitotic figures were readily apparent (a). The tumor cells were positive for CD3 (b), strongly positive for CD30 (c), and negative for ALK-1 (not shown). A diagnosis of ALCL, ALK negative was made. Eight months earlier, biopsy of the patient’s 2008 scalp lesion revealed a dense dermal infiltrate of large atypical lymphoid cells with irregular nuclei (d). The infiltrate extended to involve subcutaneous tissue and was positive for CD3 (e) and CD30 (f) and negative for ALK-1 (not shown). Subsequent TCR gene rearrangement studies supported that the prior skin biopsy represented secondary cutaneous involvement by a systemic ALK-negative ALCL
Discussion
Various factors may be involved in the pathogenesis of multiple malignancies such as age, gender, genetic predisposition, chemotherapy and/or radiation therapy, immunodeficiency, EBV infection [1], and environmental risk factors like smoking or asbestos exposure. We report the case of a patient in whom some of these factors may have been involved in the pathogenesis of his multiple malignancies. In 2003, the patient was diagnosed with lambda-positive, low-grade follicular lymphoma (Table 1) treated with three courses of FCR, resulting in a partial remission. Two years later, squamous cell carcinoma of the lung, together with DLBCL, was diagnosed (Table 1, Fig. 2a–c). The cytogenetic and flow cytometric analyses in 2003 revealed different results from those performed in 2006 (Table 2, Fig. 1a–b), supporting that the two B cell lymphomas were clonally unrelated. In 2008, a retroperitoneal lymph node biopsy again showed the presence of low-grade follicular lymphoma (Table 1, Fig. 2d–f). Flow cytometry revealed this to be lambda positive, and FISH analysis showed a BCL2 gene rearrangement, similar to the 2003 follicular lymphoma, while the DLBCL in 2006 was kappa positive with a BCL6 gene rearrangement, further substantiating two clonally distinct B cell neoplasms (Table 2, Fig. 1a–b). Finally, molecular genetic analysis confirmed that the DLBCL and the follicular lymphoma had separate clonal origins (Table 2, Fig. 4a–d). Interestingly, the patient also developed a cutaneous CD30-positive, ALK-negative peripheral T cell lymphoma that was thought to represent a primary cutaneous CD30-positive lymphoproliferative disorder (Table 1, Fig. 3d–f). However, after 8 months, biopsies of the liver and right cervical lymph node showed a peripheral T cell lymphoma consistent with ALCL, ALK negative (Table 1, Fig. 3a–c). The skin and systemic T cell lymphomas shared the same clonal TCR gene rearrangements by PCR, suggesting that both neoplasms arose from the same cell of origin (Table 2). In summary, this patient had two different B cell lymphomas, a systemic ALK-negative ALCL initially manifesting as a localized skin lesion, and squamous cell carcinoma of the lung.
Molecular genetic studies of the patient’s B cell lymphomas. IgH gene rearrangement studies on both the 2008 lymph node showing grade 1 follicular lymphoma (a and c) and the 2006 lymph node showing DLBCL (b and d) revealed clonal B cell populations. In the 2008 case, the clonal IgH gene rearrangement was detected with PCR primers to framework region I only (a, 319 bp), whereas primers to framework region II (c) showed a polyclonal B cell population. In contrast, in the 2006 case, the clonal rearrangement was detected with primers to both framework regions I (a, 323 bp) and II (d, 267 bp), indicating that the two lymphomas arose from different primaries. Framework region III primers were polyclonal in both samples (not shown)
A relationship between this patient’s different malignancies may be hypothesized based upon genetic predisposition, environmental risk factors, gender, age, and therapy administered for his initial low-grade follicular lymphoma. Lung cancer is reported to be the second most frequently diagnosed secondary malignancy following treatment for indolent non-Hodgkin’s lymphoma, after myelodysplastic syndrome or acute myeloid leukemia [8]. Factors with a statistically significant negative impact on time free from a second tumor include age 45–64 years at initial treatment, male gender and fludarabine-containing therapy. The patient we describe is a 62-year-old male former smoker with known asbestos exposure who was treated with fludarabine and who had a family history of malignancies that included lung cancer. In addition, prior therapy with fludarabine may have contributed to an immunodeficient state that allowed the growth of a previously undetected cancer [9]. Therefore, it is likely that multiple factors contributed to the development of his lung cancer.
The development of this patient's subsequent DLBCL and ALK-negative ALCL is more difficult to explain, but immunologic factors may have played a role. The squamous cell carcinoma, diagnosed in 2006, may have preceded this patient’s hematologic malignancies and remained occult, resulting in an immunological imbalance that later contributed to the development of lymphoma. Therapy with fludarabine and age-related immunosenescence may lead to the development of EBV-positive B cell lymphomas [10, 11]. However, this patient's DLBCL was EBV negative by in situ hybridization for EBV RNA (Table 1), implying that the lymphoma was unrelated to treatment with fludarabine or age. Systemic ALK-negative ALCL is not known to be associated with specific risk factors [12]. It is likely that the etiology underlying the development of multiple malignancies in this patient was multifactorial in nature and that both non-immunologic and immunologic factors (acting through a mechanism other than EBV) were involved.
This case illustrates the importance of obtaining an accurate history at the time of a patient’s initial diagnosis and of performing appropriate immunophenotypic, cytogenetic, and molecular genetic analyses during the course of a patient's diagnostic work-up. These actions help to identify important risk factors and prognostic indicators that assist in determining the most appropriate treatment strategies, taking into account possible side effects and the potential development of secondary neoplasia. Close long-term follow-up of hematologic malignancies is important not only for monitoring response to therapy but also for comparing subsequent neoplasms that may develop after the initial diagnosis to the initial pathology. The case of our patient emphasizes that new lesions developing in an individual with an established diagnosis of malignancy cannot be assumed to represent relapse of the original neoplasm. Finally, cytogenetic and molecular genetic analyses of tumors from patients with multiple malignancies may help to improve our understanding of the possible relationship between multiple primary malignancies and may help to predict the risk of a second tumor before its manifestation.
References
Dong C, Hemminkl K (2001) Second primary neoplasms among 53 159 haematolymphoproliferative malignancy patients in Sweden, 1958–1996: a search for common mechanisms. Br J Cancer 85:997–1005
Montalban C, Castrillo JM, Lopez-Abente G, Abraira V, Serrano M, Bellas C, Piris MA, Carrion R, Cruz MA, García-Larana J, Menarguez J, Rivas C (1999) Other cancers in patients with gastric MALT lymphoma. Leuk Lymphoma 33:161–168
Finnish Leukaemia Group (2000) Acute leukaemia and other secondary neoplasms in patients treated with conventional chemotherapy for multiple myeloma: a Finnish Leukaemia Group study. Eur J Haematol 65:123–127
Christou L, Tsiara S, Frangides Y, Pnevmatikos J, Briasoulis E, Galanakis E, Bourantas KL (1998) Patients with multiple myeloma and solid tumors: six case reports. J Exp Clin Cancer Res 7:239–242
Hodgson DC, Gilbert ES, Dores GM, Schonfeld SJ, Lynch CF, Storm H, Hall P, Langmark F, Pukkala E, Andersson M, Kaijser M, Joensuu H, Fossa SD, Travis LB (2007) Long-term solid cancer risk among 5-year survivors of Hodgkin's lymphoma. J Clin Oncol 25:1489–1497
Frei KA, Bonel HM, Forrer P, Alleman J, Steiner RA (2002) Primary breast lymphoma, contralateral breast cancer, and bilateral Brenner tumors of the ovary. Obstet Gynecol 100:1079–1082
Matsubayashi Y, Kakehi T, Sugiyama H et al (1986) A case of multiple myeloma associated with gastric cancer, rectal cancer and myelomatous pleural effusion in the terminal stage [Japanese]. Rinsho Ketsueki 27:2313–2318
Sacchi S, Marcheselli L, Bari A, Marcheselli R, Pozzi S, Luminari S, Lombardo M, Buda G, Lazzaro A, Gobbi PG, Stelitano C, Morabito F, Quarta G, Brugiatelli M (2008) Secondary malignancies after treatment for indolent non-Hodgkin's lymphoma: a 16-year follow-up study. Haematologica 93:398–404
Fazzi R, Caracciolo F, Galimberti S, Petrini M (2003) Early reappearance of primary solid cancer in patients treated with purine analogs. J Chemother 15:406–408
Abruzzo LV, Rosales CM, Medeiros LJ, Vega F, Luthra R, Manning JT, Keating MJ, Jones D (2002) Epstein-Barr virus-positive B cell lymphoproliferative disorders arising in immunodeficient patients previously treated with fludarabine for low-grade B cell neoplasms. Am J Surg Pathol 26:630–636
Nakamura S, Jaffe ES, Swedlow SH (2008) EBV positive diffuse large B cell lymphoma of the elderly. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW (eds) WHO classification of tumours of haematopoietic and lymphoid tissues, 4th edn. IARC, Lyon, pp 243–244
Mason DY, Harris NL, Delsol G, Stein H, Campo E, Kinney MC, Jaffe ES, Falini B (2008) Anaplastic large cell lymphoma, ALK-negative. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW (eds) WHO classification of tumours of haematopoietic and lymphoid tissues, 4th edn. IARC, Lyon, pp 317–319
Acknowledgments
The authors thank Dr. Paola Dal Cin and Ms. Cynthia McLaughlin of the Brigham and Women's Hospital Center for Advanced Molecular Diagnostics and Dr. A. John Iafrate of the Massachusetts General Hospital Molecular Diagnostics Laboratory for assistance with performance and interpretation of cytogenetic studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Cannizzo, E., Sohani, A.R., Ferry, J.A. et al. Carcinoma and multiple lymphomas in one patient: establishing the diagnoses and analyzing risk factors. J Hematopathol 2, 163–170 (2009). https://doi.org/10.1007/s12308-009-0041-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12308-009-0041-0