Abstract
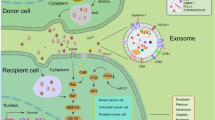
Research on the composition of the tumor micro-environment has demonstrated that membrane delimited microvesicles are shed from many types of malignant tumors, in the peripheral blood of cancer patients as well as in culture media of tumor cells propagated in vitro (Ginestra et al. Anticancer Res 18:3433–3437, 1998). Their documented effects involve the activation of signal transduction pathways by cellular cross-talk that are associated with epigenetic mechanisms that may be important in tumor progression, metastasis, and the activation of angiogenesis (Distler et al. Arthritis Rheum 52:3337–3348, 2005). Live cell imaging microscopic studies conducted in our laboratory of the formation of solid tumor spheroids in vitro show that the shedding of microvesicular structures from tumor cells occurs during this process. The observed properties of the tumor microvesicles suggest a role in solid tumor formation and intercellular communication. The tumor associated microvesicles were shown to be non-apoptotic based on the absence of fluorescent nuclear staining by acridine orange/ethidium bromide staining. Increased concentration of extracellular Ca++ [5–20 mM] resulted in an increase in the production of tumor-derived microvesicles and also to result in the formation of tumor spheroids whose size was considerably smaller than controls. Increased extracellular [Ca++] was also observed to induce the rapid dissociation of solid tumor spheroids to smaller cell aggregates in the absence of significant apoptosis.








Similar content being viewed by others
References
Ginestra A, LaPlaca MD, Saladino F et al (1998) The amount and proteolytic content of vesicles shed by human cancer cell lines correlates with their in vitro invasiveness. Anticancer Res 18:3433–3437
Distler JH, Pisetsky DS, Huber LC et al (2005) Microparticles as regulators of inflammation: novel players of cellular crosstalk in the rheumatic diseases. Arthritis Rheum 52:3337–3348
Wolf P (1967) The nature and significance of platelet products in human plasma. Br J Haematol 13:269–288
Beaudoin AR, Grondin G (1991) Shedding of vesicular material from the cell surface of eukaryotic cells: different cellular phenomena. Biochim Biophys Acta 10:171–203
Horstman LL, Jimenez JJ, Bidot C, Ahn YS (2004) New horizons in the analysis of circulating cell-derived microparticles. Keio J Med 53:210–230
Skog J, Wurdinger T, van Rijn S et al (2008) Glioblastoma microvesicles transport RNA and proteins that promote tumor growth and provide diagnostic biomarkers. Nat Cell Biol 10:1470–1476
Muralidharan-Chari V, Clancy J, Sedgwick A et al (2010) Microvesicles: mediators of extracellular communication during cancer progression. J Cell Sci 123:1603–1611
Valenti R, Huber V, Manuela I et al (2006) Tumor-released microvesicles as vehicles of immunosuppression. Cancer Res 67:2912
Tl W (2005) Tumor-derived exosomes or microvesicles: another mechanism of tumor escape from the host immune system? Br J Cancer 92:209–211
George JN, Thoi LL, McManus LM et al (1982) Isolation of human membrane microparticles from plasma and serum. Blood 60:834
Yu X, Harris SL, Levine AJ (2006) The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res 66:4795–4801
Ginestra A, Miceli D, Dolo V et al (1999) Membrane vesicles in ovarian cancer fluids: a new potential marker. Anticancer Res 19:3439–3446
DiVizio D, Kim J, Hager M et al (2009) Oncosome formation in prostate cancer: association with a region of frequent chromosomal deletion in metastatic disease. Cancer Res 69:5601–5609
Vidulescu C, Clejan S, O’Connor KC (2004) Vesicle traffic through intercellular bridges in DU 145 human prostate cancer cells. J Cell Mol Med 8:388–396, 14
Janoska-Wieczorek A, Wysoczynski M, Kijowski J et al (2005) Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer 113:3143–3149
Barry OP, Fitzgerald GA (1999) Mechanisms of cellular activation by platelet microparticles. Thromb Haemost 82:794–800
Sutherland RM (1988) Cell and environment interactions in tumor microregions: the multicell spheroid model. Science 8:177–184
Francia G, Green S, Kerbel R (2005) Epigenetic aspects of environmentally mediated “multicellular” drug resistance. Am Assoc Cancer Res Educ Book, pp 114–118
Espina V, Mariani B, Gallgher R et al (2010) Malignant precursor cells pre-exist in human breast DCIS and require autophagy for survival. PLoS ONE 5:e10240
DeRoos M, van der Vegt B, de Vries J et al (2007) Pathological and biological differences between screen-detected and interval ductal carcinoma in situ of the breast. Ann Surg Oncol 14:2097–2104, 20
Stewart AF (2005) Hypercalcemia associated with cancer. N Engl J Med 352:373–379, 21
Hotte S, Hirte H, Rabbani S, Carling T, Hendy G, Major P (2002) Hypercalcemia of malignancy: pathophysiology, diagnosis and treatment. Am J Cancer 1:179–187
Davidson TG (2001) Conventional treatment of hypercalcemia of malignancy. Am J Health-Syst Pharm 15:S8–S15, 23
Sidler B, Alpert L, Henderson JE, Deckelbaum R, Amizuka N, Silva JE, Goltzman D, Karaplis AC (1996) Amplification of the parathyroid hormone-related peptide gene in a colonic carcinoma. J Clin Endocrinol Metab 81:2841–2847
Anderson J, Grabowska A, Watson S (2007) PTHrP increases transcriptional activity of the integrin subunit α 5. Br J Cancer 96:1394–1403
Suva LJ, Winslow GA, Wettenhall RE et al (1987) A parathyroid hormone-related protein implicated in malignant hypercalcemia: cloning and expression. Science 237:893–896
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Crawford, S., Diamond, D., Brustolon, L. et al. Effect of Increased Extracellular Ca++ on Microvesicle Production and Tumor Spheroid Formation. Cancer Microenvironment 4, 93–103 (2011). https://doi.org/10.1007/s12307-010-0049-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12307-010-0049-0