Abstract
Plant responses to phosphate (Pi) starvation stress involve an array of adaptive strategies including enhanced accumulation and shoot-to-root transport of carbohydrates required for activating the plant Pi starvation signaling. However, the contribution of carbohydrate biosynthesis genes to maintaining phosphorus (P) homeostasis remains unknown, and the functional characterization of sugar metabolism genes is often impeded or compromised due to the loss of fertility of the null mutants. Here, a highly expressed gene encoding UDP-glucose pyrophosphorylase in rice (Oryza sativa), Ugp1, was functionally characterized. Ugp1 was transcriptionally induced in leaf blades by Pi starvation. As a link between sucrose (Suc) and P, we assumed that overexpression of Upg1 would alter the Suc concentration. Given that Suc is a signaling molecule, such concentration changes would affect the P deficiency signal transduction, thereby altering the homeostasis of endogenous P. The results showed that, overexpression of Ugp1 decreased plant biomass, increased sucrose content, and promoted Pi accumulation. The elevated Pi accumulation in Ugp1-OX plants was accompanied by the up-regulation of Pi-starvation-induced genes. Taken together, these results demonstrate that Ugp1 is a positive regulator of sucrose accumulation, and is required for maintaining P homeostasis in rice. Ugp1 thus represents a novel entry point to dissect the mechanisms underlying the carbon-P crosstalk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice is an extremely important cereal crop, which is the staple food for more than half of the world’s population. Currently, about 160 million hectares of paddy fields all over the world produce about 500 million tons of rice annually. However, this amount is not enough to feed the ever-growing population. In order to meet the increasing global demand, sustained efforts have been made to improve rice yield (Agarwal et al. 2016; Lau 2017).
Phosphorus (P) is one of the major macro-nutrients indispensable for rice growth and development, and is involved in important physiological and biochemical processes, such as biosynthesis, energy transfer and signal transduction. The major form of P for plants to acquire from soil is inorganic orthophosphate (Pi). Because soluble Pi is easily converted to organic form by soil microorganisms or is fixed by metal cations such as calcium, magnesium, aluminum, and iron, its mobility and availability in the soil are poor. Phosphorus is one of the main limiting factors for plant growth in farmland and natural ecosystems (Chapin 1996; Raghothama 1999). To obtain higher yields and better quality of rice, chemical P fertilizers are usually applied each year at relatively high rates, which poses a potential environmental threat and exacerbates the shortage of nonrenewable P mineral resources worldwide (Gilbert et al. 1999). Therefore, a full understanding of the mechanism of Pi signaling and absorption in rice is crucial to improve P-use efficiency (PUE). Cultivating high PUE rice varieties that effectively utilize soil P pools (including residual fertilizer P) is an unavoidable choice in the context of development of sustainable agriculture and protection of environment.
The plant Pi starvation responses show extensive interactions with other pathways, such as carbon (C) metabolism. Many plants, including rice, accumulate carbohydrates in source tissues in response to low-phosphorus stress and transport them mainly in the form of sucrose (Hammond and White 2011; Meng et al. 2020; Yoon et al. 2021). It has been reported that sucrose play a regulatory role in P deficiency signal transduction in plants (McKinley et al. 2016). Sucrose participates in the physiological processes and metabolic pathways such as root elongation, root hair growth, acid phosphatase secretion, and auxin synthesis to adapt to low Pi availability via regulating gene expression as a systemic signaling molecule (Dasgupta et al. 2014; Li et al. 2017; Zou et al. 2018). The alteration of synthesis or transport of sucrose influences the response to P starvation in plants (Hammond and White 2011). Some studies showed that inhibition of sucrose unloading in phloem could affect the response of plants to low-P stress (Lloyd and Zakhleniuk 2004; Zhang et al. 2015). Our recent results indicate that knockout of OsAGPL1 or OsAGPS1, two genes encoding the enzyme catalyzing the initial and rate-limiting step in starch biosynthesis, leads to a significant decrease in starch content and an increase in Pi accumulation. We think that it is due to a complementary increase in sucrose concentration because sucrose acts as a signal molecule influencing the P signaling pathway in rice, activates the expression of Pi-starvation-induced (PSI) genes, and finally modifies the uptake of Pi (Meng et al. 2020). However, the balance between starch and sucrose is very interesting. A change in the accumulation of either starch or sucrose may lead to an opposite or the same alteration in the other one (Miyagawa et al. 2001; Pal et al. 2013; Lee et al. 2014). To obtain robust evidence, we characterized the gene Ugp1 through bioinformatics analysis and RNA-seq results in public databases (Secco et al. 2013). This gene encodes an enzyme that participates in sucrose biosynthesis, namely uridine diphosphate-glucose pyrophosphorylase (UGPase).
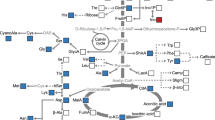
Sucrose synthesis in plants primarily occurs in the cytoplasm, using uridine diphosphate glucose (UDPG) as a precursor. Sucrose phosphate synthase (SPS) catalyzes UDPG and fructose-6-phosphate (Fru6P) to produce sucrose-6-phosphate, which is then hydrolyzed to form sucrose (Fig. 1). UDPG is crucial in various cellular process. Apart from serving as a precursor for sucrose, it is essential for cellulose, callose, and other cell wall polysaccharide synthesis (Kleczkowski et al. 2010; Bar-Peled and O’Neill 2011; Janse van Rensburg and Ende 2017). Additionally, UDPG acts as a glucose donor during the synthesis of glycoproteins, glycolipids, and sulfolipids (Okazaki et al. 2009). UDPG is known to participate in approximately 270 plant reactions (Chae et al. 2014). There are two enzymes, UGPase and uridine diphosphate-sugar pyrophosphorylase (USPase), that catalyze the reversible reaction using glucose-1-phosphate (Glc1P) as a substrate to synthesize UDPG (UTP + Glc1P ↔ UDPG + PPi) (Kleczkowski et al. 2010; Yin et al. 2011). In source tissues, UGPase primarily catalyzes UDPG synthesis. In mature Arabidopsis leaves (based on studies with UGPase mutants), UGPase accounts for at least 90–94% of UDPG-dependent activity (Park et al. 2010; Janse van Rensburg and Ende 2017). There are two types of plant UGPase, type-A and type-B. Type-A UGPase is typically cytoplasmic (Okazaki et al. 2009; Kleczkowski et al. 2010) and is particularly important in storage tissues (e.g., endosperm and tuber) and reproductive organs (Sowokinos et al. 1993; Spychalla et al. 1994). Some type-B UGPase enzymes localize to plastids and provide UDPG for sulfolipid biosynthesis (Okazaki et al. 2009). Consequently, UGPase plays a critical role in plant growth and development.
Main pathways of starch and sucrose synthesis in plant cells. Purple fonts indicate the enzymes involved in UDPG and sucrose formation. Arrows connect respective substrates to products. Abbreviations: Triose-P: Triose phosphate; Fru6P: Fructose-6-phosphate; Glc6P: Glucose-6-phosphate; Glc1P: Glucose-1-phosphate; ADPG: Adenosine diphosphate glucose; UDPG: Uridine diphosphate glucose; Suc: Sucrose; UGPase: UDPG pyrophosphorylase; USPase: UDP-sugar pyrophosphorylase; SPP: Sucrose phosphate phosphatase; SPS: Sucrose phosphate synthase
In rice, two UGPase homologs, Ugp1 (LOC_Os09g38030) and Ugp2, encode type-A UGPase (Abe et al. 2002; Mu et al. 2009). Ugp1 is abundantly expressed in pollen cells and maintains rice fertility by promoting callose deposition during the meiosis stage of pollen development (Chen et al. 2007b; Woo et al. 2008). Ugp2 shows a much lower abundance compared with Ugp1, and is preferentially expressed in pollen (Chen et al. 2007b; Mu et al. 2009). Based on the bioinformatics analysis of Ugp1 and the results of preliminary studies on the interaction between the carbon and P metabolic pathways (Chen et al. 2007a; Meng et al. 2020), it is hypothesized that Ugp1 may participate in the P signaling pathway by (i) regulating the metabolic balance of endogenous carbohydrates, and (ii) influencing the crosstalk between the P and carbon metabolic pathways in rice. In this study, we overexpressed Ugp1 in rice, and analyzed the growth, development, carbohydrate accumulation, and P acquisition of transgenic plants in different P supply conditions.
Materials and methods
Plant materials and growth conditions
A japonica variety (Nipponbare) of rice (Oryza sativa L. ssp. japonica) was used as the wild-type (WT). The transgenic plants and WT plants were grown in hydroponics providing standard rice culture nutrition as: 1.25 mM NH4NO3, 0.2 mM NaH2PO4, 0.513 mM K2SO4, 0.998 mM CaCl2, 1.643 mM MgSO4, 0.009 mM MnCl2, 0.075 mM (NH4)6Mo7O24, 0.019 mM H3BO3, 0.155 mM CuSO4, 0.02 mM Fe-EDTA, 1 mM NaSiO3, and 0.152 mM ZnSO4. Rice plants were grown in a growth chamber with 14 h light/12 h dark photoperiod, day/night temperatures of 30/22 °C, and the relative humidity was controlled at approximately 60%. Seedlings were treated with 0.5 strength of the nutrient solution described above until the fourth leaf blade just emerged. Subsequently, the seedlings were treated with the full-strength solution containing high phosphate (HP; 200 μM Pi; as detailed above) or low phosphate (LP; 10 μM Pi; 0.01 mM NaH2PO4) for 3 weeks during the vegetative stage, and then evaluated for phenotype or sampled.
RNA extraction, cDNA synthesis and RT-qPCR
Total RNA was extracted from different plant tissues such as leaf blades, leaf sheaths and roots, using TRIzol reagent (Invitrogen). For synthesizing first-strand cDNAs from total RNA, we used a PrimeScript RT reagent kit with gDNA Eraser (TaKaRa Biotechnology, Dalian, China) according to the manufacturer’s instructions. Real time–quantitative PCR (RT-qPCR) was performed with a SYBR Premix Ex Taq™ II kit (TaKaRa Biotechnology) on a StepOnePlus Real-Time PCR System (Applied Biosystems) following the manufacturer’s instructions. Relative expression of the target genes was calculated against that of OsActin1 (LOC_Os03g50885) with the 2−△CT or 2−△△CT method. All PCR primers used in this study are listed in Tables S1 and S2.
Expression, purification, SDS/PAGE, and enzyme activity assay of UGPase
The Ugp1 cDNA encoding sequence was subcloned into the pET-29a( +) vector (Novagen). The construct was transformed into Escherichia coli BL21/DE3 (pLysS) cells, and protein expression was optimized. The cells were grown to the late exponential phase (OD600 ~ 0.8) in conical flasks containing LB medium with 25 μg/mL kanamycin and 25 μg/mL chloromycetin. Recombinant protein expression was induced by the addition of isopropyl-d-thiogalactopyranoside (IPTG) to the final concentration of 1 mM. Cells were grown for another 8 h at 20 °C, and then centrifuged at 8000 g at 4 °C for 10 min. The pellet was resuspended in the binding buffer containing 20 mM Na3PO4, 500 mM NaCl and 5 mM imidazole. The homogenate was centrifuged at 10,000 g and 4 °C for 10 min, and the resulting supernatant was used to assay UGPase activity. The recombinant proteins were purified using a 6 × His-Tagged Protein Purification kit (CWBIO, CW0894S) based on manufacturer’s instructions. The protein concentrations were determined by a BCA Protein Assay kit (GenStar, E162-01).
The cell pellet or purified protein mixed with SDS loading buffer (Takara), was boiled for 10 min and then centrifuged at 10,000g for 5 min. The supernatant was loaded on a 12% SDS–polyacrylamide gel. After electrophoresis, the gel was stained with Coomassie blue. For Western blot analysis, proteins were transferred to a nitrocellulose membrane after electrophoresis. Membranes were blocked in 5% v/v non-fat milk in TBS-T (20 mM Tris–HCl, pH 7.6, 137 mM NaCl, and 0.1% w/w Tween 20) at room temperature for 1 h. For detection of recombinant protein, the membranes were incubated with an anti-6 × HIS antibody (CMCTAG, AT0025) at 1:5000 dilution with TBS-T at 4 °C overnight. Next, the membranes were washed in TBS-T three times for 10 min each. Membranes were then incubated for 1 h with a goat anti-mouse lgG (H + L) cross-adsorbed secondary antibody (Invitrogen, 35519) at 1:10,000 dilution in TBS-T, followed by five washes for 6 min each in TBS-T, and detection using DyLight 680.
The UGPase activity assay was performed with the recombinant proteins using a one-step spectrophotometric method (Sowokinos et al. 1993). The formation of NADPH was monitored at 37 °C until the maximum change in optical density occurred at 340 nm. The difference in absorbance before and after the addition of the supernatant was used to calculate the amount of NADPH generated. One unit of activity in the synthesis direction is defined as the amount of enzyme required to catalyze the production of 1 nmol of NADPH per minute.
Vector construction for Ugp1 overexpression, and generation of transgenic plants
For Ugp1 overexpression, the GUSPlus reporter gene was introduced into pCAMBIA1305 via NcoI/BglII digestion, resulting in a new expression vector, pCAMBIA1305-GUSPlus. The Ugp1 cDNA encoding sequence was amplified and fused upstream of the GUSPlus reporter gene via SacI/BamHI. The constructs were introduced into Agrobacterium tumefaciens (strain EHA105) by electroporation. The Agrobacterium-mediated transformation was performed using mature embryos developed from seeds of WT rice plants following the standard procedure (Jia et al. 2011).
Measurement of carbohydrates
Shoots and roots of WT and transgenic plants were sampled separately and dried. A sample of 0.1 g was extracted in 10 mL distilled water at 100 °C for 30 min. The extract was filtered into a 25 mL volumetric bottle, and the reaction mixture, containing 0.5 mL of extract, 1.5 mL of distilled water, 0.5 mL of 2% (w/v) anthrone with ethylacetate and 5 mL of 18 M H2SO4, was incubated in boiling water for 1 min. The washed precipitate, after digestion with amyloglucosidase, was used for starch estimation (Fredeen et al. 1989). Sucrose was estimated as described previously (Jelitto et al. 1992). All determinations were performed in four to six replicates from which standard deviation was calculated.
Measurement of soluble Pi and total P concentration
Shoots and roots of WT and transgenic plants were sampled separately. For the measurement of soluble Pi concentration, about 0.5 g of fresh samples were used following the method below (Zhou et al. 2008). Soluble Pi was extracted by perchloric acid and determined by the molybdenum blue method. For the measurement of total P concentration, about 0.1 g of dry samples were used following the method described elsewhere (Chen et al. 2007a). The dried plant tissues were ground (average diameter 1 mm) and then digested with a mixture of 5 mL of 98% w/w H2SO4 and 3 mL of 30% v/v hydrogen peroxide. After cooling, the digested sample was diluted to 100 mL with distilled water. The total P concentration in the solution was measured using the molybdenum blue method with absorbance read at 820 nm on a SpectraMax M5 multidetection microplate reader system.
Phosphorus uptake using 32P isotope
To perform the Pi uptake assay, 10-day-old WT and transgenic plants were treated with HP or LP nutrient solution for 7 days before 32P uptake assay. The plant roots were first incubated in a pretreatment solution (2 mM MES and 0.5 mM CaCl2, pH 6.0) before transferring them into a 1 L uptake solution (nutrient solution specified above plus 100 μM NaH2PO4, pH 6.0) containing [32P] orthophosphate (PerkinElmer) (8 µCi L−1) for 5 min, 30 min and 3 h. At the end of the uptake period, roots were washed three times with double-distilled H2O, and then were transferred into ice-cold desorption solution (2 mM MES, 0.5 mM CaCl2, 100 μM NaH2PO4, pH 6.0) for 15 min. Afterwards, the roots were blotted, plants were cut into shoots and roots with a razor, weighed, and placed in 10 mL centrifuge vials with 1 mL of perchloric acid and 0.5 mL of hydrogen peroxide. The vials containing samples were then digested in an oven at 65 ℃ overnight. Subsequently, 0.2 mL of the digest was transferred into a 5 mL vial, 3 mL of scintillation cocktail (ULTIMA GOLD™ LLT; PerkinElmer) was added to each vial, and radioactivity was measured by a scintillation counter (Beckman Coulter LS6500).
Results
Ugp1 was specifically induced by phosphate deficiency and showed UGPase activity in vitro
We screened the reported transcriptomic data and found that Ugp1 was up-regulated in rice shoots by Pi starvation stress. For validations, RT-qPCR analysis was performed to analyze the transcriptional response of Ugp1 to major nutrient deficiencies. The results confirmed that Ugp1 expression was induced by Pi starvation in leaves but not in roots. This transcriptional induction was not observed upon deprivation of nitrogen, potassium, magnesium or iron (Fig. 2).
The Expression Pattern of Ugp1. The expression of Ugp1 in response to deficiency of various nutrients. RT-qPCR was performed on total RNAs from the 5th leaf blade and root of 15-d-old seedlings after growth in nutrient sufficient (C), phosphate-deficient (-P), nitrate-deficient (-N), potassium-deficient (-K), iron-deficient (-Fe) and magnesium-deficient (-Mg) conditions for 10 d. Values represent means with SE of three biological replicates. Different letters indicate significant differences (P < 0.05, Duncan’s t-test)
The Upg1 cDNA encoding sequence was firstly subcloned into the pET-29a vector and then transformed into E. coli for enzyme activity assays. The empty vector was used as a negative control. The expression of His-tagged recombinant protein was induced by adding IPTG, and the protein was purified and separated by SDS-PAGE (Fig. 3a). Western blot analysis was used to confirm whether the recombinant protein was expressed successfully in vitro (Fig. 3b). The UGPase activity assay was performed with recombinant protein by monitoring the NADPH formation at 340 nm. As expected, after the addition of IPTG, the Ugp1 recombinant protein was successfully expressed in E. coli. Furthermore, the enzyme activity of the recombinant protein was detected, whereas the empty vector protein had no enzymatic activity, indicating that the recombinant protein was functional (Fig. 3c). In recombinant protein, the UGPase enzyme activity was 0.14 nmol NADPH/min, which was approximately 28 times that of the empty vector protein.
Expression, Purification and Enzyme Activity Assay of Ugp1 in E. coli. a SDS-PAGE of empty vector (EV) and recombinant proteins induced with ( +) or without ( −) IPTG (left) and purified recombinant Ugp1 protein (right). The molecular weight of the His-tagged vector (6.3 kDa) recombinant Ugp1 protein agrees with the estimated value of 51.6 kDa for the full-length Ugp1 protein (469 aa). b Western blot analysis of EV and purified recombinant proteins induced with ( +) or without ( −) IPTG. The proteins separated by SDS-PAGE were transferred to the PVDF membrane, and the membrane was incubated with His-tagged antibody and lgG (H + L) antibody successively. The specific bands could be observed at 680 nm (upper panel) or after Ponceau S staining (lower panel). c Activity assays of recombinant Ugp1 protein. The formation of NADPH was calculated from the absorption changes at 340 nm monitored after 4 h using an NADPH molar extinction coefficient of 6.22 × 103 M−1 cm−1
Identification of Ugp1-overexpressing transgenic rice plants
To elucidate the role of Ugp1 in rice growth and development, we overexpressed the Ugp1 gene under the control of the CaMV 35S promoter in the pCAMBIA1305.1 vector (Fig. 4a). About 40 independent transgenic plants (Ugp1-OX) were produced in T0 generation (Fig. S1). Ugp1-OX T0 plants with a high Ugp1 expression level (about 10 lines) were selected for further characterization. T1 lines were germinated and transplanted in the field in Nanjing. Three lines selected randomly were used for seeding, named OX15, OX30 and OX36. When the seedlings grew to the four-leaf-stage in a controlled-environment chamber, samples of leaf blades were collected, and RNA was extracted to identify the abundance of Ugp1 transcripts in transgenic plants (Fig. 4b). The results showed that the Ugp1 expression significantly increased in these three lines. The expression levels of Ugp1 in the OX15, OX30, and OX36 lines were 3.3, 4.3, and 3.7 times higher than that of WT rice, respectively. We also performed UGPase activity assays in transgenic plants (Fig. 4c). Compared with WT, Ugp1-OX T1 plants showed a 2.5-fold higher induction of UGPase activity in leaves.
Ugp1 Expression in transgenic rice plants. a Structure of Ugp1-overexpressing construct for rice transformation. The construct was developed under the control of the 35S promoter and the nopaline synthase (nos) terminator cassette. b Relative expression level of Ugp1 in Ugp1-OX T1 generation transgenic plants. The data are means with SE of three biological replicates. c UGPase activity in transgenic plants. UGPase activities are shown as means with SE of three biological replicates
Overexpressing Ugp1 affects rice growth and development
When Ugp1-OX transgenic plants were grown in soil until ripening, they displayed a phenotype of significantly shorter plant height than wild type (Fig. S2). However, this phenotype did not appear during the vegetative stage. To identify the influence of Ugp1 on rice growth during vegetative stage, three independent Ugp1-OX lines (OX15, OX30 and OX36) were used to detect the biomass and length of shoots and roots in HP (200 μM Pi) and LP (10 μM Pi) conditions (Fig. 5a). Biomass of Ugp1-OX shoots was decreased in both HP and LP conditions in comparison with WT (Fig. 5b). Similarly, a decrease in root biomass was observed in the Ugp1-OX lines (Fig. 5c). In addition, it was observed that treatment with LP significantly promoted the growth of roots and had an inhibitory effect on shoots growth in both WT rice and Ugp1-OX lines, resulting in a significant increase in root-to-shoot ratio (Fig. 5b, c).
Phenotype of Ugp1-overexpressing plants. a Phenotype of Ugp1-overexpressing plants grown in nutrient solution supplied with different concentrations of Pi (HP, 200 µM Pi; and LP, 10 µM Pi) for 3 weeks. b, c Biomass of shoots and roots in Ugp1-OX lines under HP and LP supply. DW, dry weight. Values represent means with SE of four biological replicates, **P < 0.01, Duncan’s t-test
Carbohydrate allocation is altered in Ugp1-overexpressing plants
The concentrations of sucrose and starch were determined in the roots and shoots of Ugp1-OX plants after 3-weeks of growth in HP and LP conditions to examine the effects of Ugp1 overexpression on internal sugar levels (Fig. 6). Sucrose concentration was significantly higher in transgenic plants than WT plants grown with sufficient Pi supply. Interestingly, such an increase did not occur in the LP treatment. No change was observed in starch concentration regardless of HP or LP supply.
Carbohydrate Concentration in Ugp1-overexpressing plants. a, b Sucrose concentration of shoots and roots in Ugp1-OX transgenic plants under HP (200 μM) and LP (10 μM) supply. c, d Starch concentration in shoots and roots of Ugp1-OX transgenic plants under HP and LP supply. The seedlings were grown hydroponically for 3 weeks. DW, dry weight. Values represent means with SE of six biological replicates, *P < 0.05, **P < 0.01, Duncan’s t-test
Ugp1 affects Pi uptake and accumulation in rice
To investigate the potential role of Ugp1 in maintaining Pi homeostasis, Pi concentration and 32P-labeled Pi uptake rate were detected in Ugp1-OX lines. Three-leaf transgenic and WT plants were grown in a hydroponic system with different Pi supplies for 3 weeks. Compared with WT plants, a significant increase in the short-term Pi uptake rate by Ugp1-OX roots as well as Pi accumulation in shoots was observed under HP but not LP supply, and the difference gradually increased over time (Fig. 7). After 24 h, Pi uptake rate of both roots and shoots in Ugp1-OX was more than1.4-fold higher compared to WT lines. Correspondingly, Pi concentration in shoots and roots was markedly higher in Ugp1-OX than WT plants when supplied with sufficient Pi (Fig. 8). Compared with WT plants, the concentrations of total P as well as inorganic Pi in transgenic lines were significantly (23 and 31%, respectively) higher in all tissues (especially in roots under HP supply). Under low P supply, the concentrations of total P and Pi did not differ between Ugp1-OX and WT plants. The results suggested a positive correlation between transcript levels of Ugp1 and P concentration in rice treated with HP supply.
Pi Uptake rate in Ugp1-overexpressing plants. Pi uptake by roots and shoots of Ugp1-OX and WT plants. The seedlings were grown with Pi-sufficient (HP, 200 µM Pi) and Pi-deficient supply (LP, 10 µM Pi) for 3 weeks, and placed in a hydroponic system containing 100 µM Pi labeled with radioactive 32P. The Pi uptake were assessed after 3, 8 and 24 h. Values presented are means with SE of three biological replicates. FW, fresh weight. **P < 0.01, Duncan’s t-test
Total Phosphorus and Inorganic Phosphate Concentration in Ugp1-overexpressing plants. a Total P concentration of shoots and roots in Ugp1-OX lines and WT. b Pi concentration of shoots and roots in Ugp1-OX lines and WT. The plants were grown in the HP (200 µM Pi) and LP (10 µM Pi) nutrient solutions for 3 weeks. Values presented are means with SE of six biological replicates (A) and four biological replicates (B). DW, dry weight. FW, fresh weight. *P < 0.05, **P < 0.01, Duncan’s t-test
Overexpression of Ugp1 impacts the expression of PHT1 genes
Ugp1-OX transgenic plants showed excellent Pi uptake capacity and thus accumulated more P than WT plants in the HP treatment. Did PHT1 genes play a role in this process? To clarify the influence of Ugp1 overexpression on PHT1 genes, the expression patterns of several major OsPHT1 genes were quantified (Fig. 9). As expected, the expression of all the detected genes (except PHT1;6) was induced in the Ugp1-OX lines when supplied with sufficient Pi (Fig. 9a). In particular, the expression of PHT1;1 and PHT1;8 (the two genes that play a crucial role in maintaining P homeostasis in rice) was up-regulated in transgenic plants in contrast to WT. Interestingly, although Pi uptake rate and Pi concentration in Ugp1-OX transgenic plants showed no significant difference compared to WT plants in LP conditions, there was a significant difference in the expression of PHT1 genes. The OsPHT1 genes were down-regulated (by at least 50%) in Ugp1-OX plants (Fig. 9b). These results suggested that Ugp1 may participate in the response to Pi starvation by regulating the PHT1 genes expression at the transcriptional level in normal P (HP) supply conditions.
Expression of Genes Involved in Pi Transport and Signaling in Ugp1-overexpressing plants. The expression of major PHT1 genes was measured in roots of Ugp1-OX transgenic lines and WT after treatment in the HP (200 µM Pi, a) and LP (10 µM Pi, b) nutrient solutions. Values presented are means with SE of three biological replicates. **P < 0.01, Duncan’s t-test
Discussion
Ugp1 is a major UGPase for sucrose biosynthesis in rice
Sucrose is the main product of photosynthesis in plants. As a key enzyme in the sucrose synthesis pathway, UGPase has been studied thoroughly in various species from plants to microorganisms since 1953 (Munch et al. 1953; Sowokinos et al. 1993; Elling and Kula 1994). In the present study, we found that Ugp1 catalyzed the synthesis of UDPG in vitro, exhibiting UGPase activity (Fig. 3); the Ugp1 overexpression resulted in increased sucrose accumulation in rice shoots and roots (Fig. 6). Similarly, overexpression of LgUGPase increased soluble sugar content in Arabidopsis (Mei et al. 2018). In addition, sucrose content in the atugp1/atugp2 double mutant plants was decreased significantly compared to the wild type in Arabidopsis (Park et al. 2010). However, it is interesting to note that overexpression of the bacterial UGPase gene in hybrid poplar did not increase soluble sugar content (Coleman et al. 2007), which was likely due to the significant differences between the prokaryotic and eukaryotic UGPase genes, and the bacterial UGPase gene did not complement the function of poplar UGPase. In addition, the increase of sucrose concentration in leaf blades indirectly down-regulated the expression of a number of genes that encode the subunits and small subunits in the photosynthetic system, thus inhibiting photosynthesis (Hermans et al. 2006; Rook et al. 2006; Morcuende et al. 2007). This might explain the restricted growth of Ugp1-OX plants (Fig. 5). In summary, Ugp1 plays an important role in carbohydrate metabolism involving sucrose biosynthesis; thus, overexpression of Ugp1 can increase the endogenous sucrose content in rice.
Intracellular sucrose is vital for maintaining P homeostasis in rice
In the past two decades, the mechanism of plant response to P deficiency signaling has been studied intensely, and sucrose plays an important role as a signaling molecule. The Ugp1 is strongly and specifically induced in rice leaf blades by P deficiency but not by deficiency of other nutrients (nitrogen, potassium, iron, and magnesium) (Fig. 2). The AtUgp1, the homologous gene of Ugp1 in Arabidopsis, can also be induced by low P. Given that the other product (in addition to UDP-Glc) of the reaction catalyzed by UGPase, namely PPi, could be further digested to Pi by pyrophosphatase (Segami et al. 2018), it could be postulated that the up-regulation of the Ugp1 expression by P starvation can be considered as the biochemical regulatory mechanism for increasing Pi in plants.
P-starvation responses in plants involve increased sucrose content in source tissues. This accumulated sucrose in leaf blades can enhance the expression of SWEETs responsible for transporting sucrose to the roots. Finally, sucrose in roots can act as a systemic signal to regulate the expression of specific genes (Hermans et al. 2006; Nilsson et al. 2010; George et al. 2011). Once sucrose synthesis or transport is altered, the response of plants to P starvation can also change (Hammond and White 2011). When supplied with sufficient Pi, the 32P-labeled isotope absorption showed that Pi uptake as well as total P and Pi concentration was significantly higher in Ugp1-OX transgenic plants than in WT (Figs. 7, 8). The expression of major members of the OsPHT1 family was detected through RT-qPCR, and the results showed that OsPHT1;1 and OsPHT1;8 were significantly up-regulated (Fig. 9a). These two genes are expressed almost constitutively in rice, and both participate in the uptake of Pi by roots and the transport and distribution of Pi in different tissues regardless of the level of P supply, playing a key role in maintaining P homeostasis in plants (Jia et al. 2011; Sun et al. 2012). In the presence of sufficient P supply, overexpression of Ugp1 significantly promoted sucrose accumulation in rice shoots, increasing sucrose transport to and concentration in the roots. Given that carbohydrate concentration in root is crucial for responding to P starvation signals, the up-regulation of PHT1 family genes followed, thereby promoting Pi uptake, transport and accumulation. This hypothesis is consistent with the results on Arabidopsis. The athps1 mutants accumulated excessive sucrose in shoots and roots, making the mutant display many characteristics of the low-P response even when treated with sufficient P, including increased acid phosphatase activity, increased anthocyanin content, promoted lateral root development, and inhibited primary root elongation. In addition, about 73% of genes induced by P starvation in WT plants were up-regulated in the athps1 mutant under sufficient P supply (Lei et al. 2011).
We found that the background expression level of Ugp1 in WT plants was very high, with approximately 10 times the expression level of OsActin (OsRac1, AB047313) in leaf blades and more than 4 times in roots (Fig. 2). In addition, when subjected to P deficiency, the Ugp1 expression was induced by more than twofold compared with the sufficient P supply. The expression level of Ugp1 was not completely linear with sucrose concentration. Studies have shown a threshold of UGPase activity for normal growth in the range of 6–25% of WT UGPase activity (Meng et al. 2009; Park et al. 2010). We also found a threshold for increasing UGPase activity, potentially explaining why overexpression of Ugp1 did not alter the sucrose concentration under low-P stress (Fig. 6). When calculating the extent of increased carbohydrate concentration under P deficiency, we found that sucrose concentration in shoots of Ugp1-OX plants increased by 59% when treated with LP nutrient solution compared with the HP treatment, whereas this increase in WT rice was 100% (Fig. 6). This indicates that overexpression of Ugp1 affects, at least partially, the response of rice to low P stress. The same result was also observed in roots as the sink tissue. The expression of tested OsPHT1 family genes was down-regulated to varying degrees under LP supply (Fig. 9b). Considering the significant decrease in biomass of Ugp1-OX plants (Fig. 5), the unchanged Pi uptake rate and P concentration per unit weight might have been compensated for by biomass reduction (Figs. 7, 8). In summary, changing the expression of Ugp1 affects the P homeostasis in rice, with Ugp1 overexpression leading to P accumulation in rice under sufficient P supply.
It should be borne in mind that overexpression is not a usual approach for identifying gene function. This method was chosen due to the difficulty in obtaining Ugp1 mutants. More convincing evidence is still needed to characterize Ugp1 function. Moreover, due to the presence of Ugp2, the highly homologous gene to Ugp1 in rice genome (Mu et al. 2009), further research remains to be done to determine whether Ugp1 overexpression affects the expression and function of Ugp2, which in turn would influence the physiological and metabolic processes in rice.
References
Abe T, Niiyama H, Sasahara T (2002) Cloning of cDNA for UDP-glucose pyrophosphorylase and the expression of mRNA in rice endosperm. Theor Appl Genet 105:216–221. https://doi.org/10.1007/s00122-002-0927-z
Agarwal P, Parida SK, Raghuvanshi S, Kapoor S, Khurana P, Khurana JP et al (2016) Rice improvement through genome-based functional analysis and molecular breeding in India. Rice 9(1):1–17. https://doi.org/10.1186/s12284-015-0073-2
Bar-Peled M, O’Neill MA (2011) Plant nucleotide sugar formation, interconversion, and salvage by sugar recycling. Annu Rev Plant Biol 62:127–155. https://doi.org/10.1146/annurev-arplant-042110-103918
Chae L, Kim T, Nilo-Poyanco R, Rhee SY (2014) Genomic signatures of specialized metabolism. Science 344(6183):510–513. https://doi.org/10.1126/science.1252076
Chapin FS (1996) Phosphorus in the global environment: transfers, cycles and management. Geoderma 73(3):257–258. https://doi.org/10.1016/0016-7061(96)00046-8
Chen AQ, Hu J, Sun SB, Xu GH (2007a) Conservation and divergence of both phosphate- and mycorrhiza-regulated physiological responses and expression patterns of phosphate transporters in solanaceous species. New Phytol 173:817–831. https://doi.org/10.1111/j.1469-8137.2006.01962.x
Chen R, Zhao X, Shao Z, Wei Z, Wang Y, Zhu L et al (2007b) Rice UDP-glucose pyrophosphorylase1 is essential for pollen callose deposition and its cosuppression results in a new type of thermosensitive genic male sterility. Plant Cell 19:847–861. https://doi.org/10.1105/tpc.106.044123
Coleman HD, Canam T, Kang KY, Ellis DD, Mansfield SD (2007) Over-expression of UDP-glucose pyrophosphorylase in hybrid poplar affects carbon allocation. J Exp Bot 58:4257–4268. https://doi.org/10.1093/jxb/erm287
Dasgupta K, Khadilkar AS, Sulpice R, Pant B, Scheible WR (2014) Expression of sucrose transporter cDNAs specifically in companion cells enhances phloem loading and long-distance transport of sucrose but leads to an inhibition of growth and the perception of a phosphate limitation. Plant Physiol 165(2):715–731. https://doi.org/10.1104/pp.114.238410
Elling L, Kula MR (1994) Purification of UDP-glucose pyrophosphorylase from germinated barley (malt). J Biotechnology 34:157–163. https://doi.org/10.1016/0031-9422(96)00089-1
Fredeen AL, Rao IM, Terry N (1989) Influence of phosphorus nutrition on growth and carbon partitioning in Glycine max. Plant Physiol 89(1):225–230. https://doi.org/10.2307/4271817
George TS, Fransson AM, Hammond JP, White PJ (2011) Phosphorus nutrition: rhizosphere processes, plant response and adaptations. Soil Biology 100:245–271. https://doi.org/10.1007/978-3-642-15271-9_10
Gilbert GA, Knight JD, Vance CP, Allan DL (1999) Acid phosphatase activity in phosphorus deficient white lupin roots. Plant Cell Environ 22:801–810. https://doi.org/10.1046/j.1365-3040.1999.00441.x
Hammond JP, White PJ (2011) Sugar signaling in root responses to low phosphorus availability. Plant Physiol 156:1033–1040. https://doi.org/10.1104/pp.111.175380
Hermans C, Hammond JP, White PJ, Verbruggen N (2006) How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci 11(12):610–617. https://doi.org/10.1016/j.tplants.2006.10.007
Janse van Rensburg HC, Van den Ende W (2017) UDP-Glucose: a potential signaling molecule in plants? Front Plant Sci 8:2230. https://doi.org/10.3389/fpls.2017.02230
Jelitto T, Sonnewald U, Willmitzer L, Hajirezeai M, Stitt M (1992) Inorganic pyrophosphate content and metabolites in potato and tobacco plants expressing E. coli pyrophosphatase in their cytosol. Planta 188(2):238–244. https://doi.org/10.1007/bf00216819
Jia HF, Ren HY, Gu M, Zhao JN, Sun SB, Zhang X et al (2011) The phosphate transporter gene OsPht1;8 is involved in phosphate homeostasis in rice. Plant Physiol 156:1164–1175. https://doi.org/10.1104/pp.111.175240
Kleczkowski LA, Kunz S, Wilczynska M (2010) Mechanisms of UDP-glucose synthesis in plants. Plant Sci 29:191–203. https://doi.org/10.1080/07352689.2010.483578
Lau W (2017) The challenges of rice sustainability: the increasing world’s population and climate change. http://blogs.springeropen.com/springeropen/2017/04/19/challenges-rice-sustainability-increasing-worlds-population-climate-change/. Accessed 20 June 2022
Lee SK, Eom JS, Voll LM, Prasch CM, Park YI, Hahn TR et al (2014) Analysis of a triose phosphate/phosphate translocator-deficient mutant reveals a limited capacity for starch synthesis in rice leaves. Mol Plant 7:1705–1708. https://doi.org/10.1093/mp/ssu082
Lei MG, Liu YD, Zhang BC, Zhao YT, Wang XJ, Zhou YH et al (2011) Genetic and genomic evidence that sucrose is a global regulator of plant responses to phosphate starvation in Arabidopsis. Plant Physiol 156:1116–1130. https://doi.org/10.4161/psb.6.8.16378
Li Y, Wang YN, Zhang H, Zhang Q, Zhai H, Liu QC et al (2017) The plasma membrane-localized sucrose transporter IbSWEET10 contributes to the resistance of sweet potato to Fusarium oxysporum. Front Plant Sci 8(e44467):197. https://doi.org/10.3389/fpls.2017.00197
Lloyd JC, Zakhleniuk OV (2004) Responses of primary and secondary metabolism to sugar accumulation revealed by microarray expression analysis of the Arabidopsis mutant, pho3. J Exp Bot 400:1221–1230. https://doi.org/10.1093/jxb/erh143
McKinley B, Rooney W, Wilkerson C, Mullet J (2016) Dynamics of biomass partitioning, stem gene expression, cell wall biosynthesis, and sucrose accumulation during development of Sorghum bicolor. Plant J 88(4):662–680. https://doi.org/10.1111/tpj.13269
Mei LL, Guo X, Zhang Y, Lin XF, Zhang WB (2018) Cloning of promoter sequence of UDP pyrophosphorylase gene from Larix gmelinii and construction of binary vector. Molecular Plant Breed 16(22):7336–7342. https://doi.org/10.13271/j.mpb.016.007336
Meng M, Geisler M, Johansson H, Harholt J, Scheller HV, Mellerowicz EJ et al (2009) UDP-glucose pyrophosphorylase is not rate limiting, but is essential in Arabidopsis. Plant Cell Physiol 50:998–1011. https://doi.org/10.1093/pcp/pcp052
Meng Q, Zhang WQ, Hu X, Shi XY, Chen LL, Dai XL et al (2020) Two ADP-glucose pyrophosphorylase subunits, OsAGPL1 and OsAGPS1, modulate phosphorus homeostasis in rice. Plant J 104:1269–1284. https://doi.org/10.1111/tpj.14998
Miyagawa Y, Tamoi M, Shigeoka S (2001) Overexpression of a cyanobacterial fructose-1,6-/sedoheptulose-1,7-bisphosphatase in tobacco enhances photosynthesis and growth. Nat Biotechnol 19(10):965–969. https://doi.org/10.1038/nbt1001-965
Morcuende R, Bari R, Gibon Y, Zheng K, Datt W, Pant B et al (2007) Genome-wide reprogramming of metabolism and regulatory networks of Arabidopsis in response to phosphorus. Plant Cell Environ 30:85–112. https://doi.org/10.1111/j.1365-3040.2006.01608.x
Mu H, Ke J, Liu W, Zhuang C, Yip W (2009) UDP-glucose pyrophosphorylase2 (OsUgp2), a pollen-preferential gene in rice, plays a critical role in starch accumulation during pollen maturation. Chin Sci Bull 54:234–243. https://doi.org/10.1007/s11434-008-0568-y
Munch PA, Kalckar HM, Cutolo E, Smith EB (1953) Enzymatic production of uridine triphosphate:uridine diphos phoglucose pyrophosphorolysis. Nature 172:1036–1037. https://doi.org/10.1038/1721036a0
Nilsson L, Müller R, Nielsen TH (2010) Dissecting the plant transcriptome and the regulatory responses to phosphate deprivation. Plant Physiol 139:129–143. https://doi.org/10.1111/j.1399-3054.2010.01356.x
Okazaki Y, Shimojima M, Sawada Y, Toyooka K, Narisawa T, Mochida K et al (2009) A chloroplastic UDP-glucose pyrophosphorylase from Arabidopsis is the committed enzyme for the first step of sulfolipid biosynthesis. Plant Cell 21:892–909. https://doi.org/10.1016/j.disopt.2008.10.003
Pal SK, Liput M, Piques M, Ishihara H, Obata T, Martins MCM et al (2013) Diurnal changes of polysome loading track sucrose content in the rosette of wild-type Arabidopsis and the starchless pgm mutant. Plant Physiol 162(3):1246–1265. https://doi.org/10.2307/41943476
Park JI, Ishimizu T, Suwabe K, Sudo K, Masuko H, Hakozaki H et al (2010) UDP-glucose pyrophosphorylase is rate limiting in vegetative and reproductive phases in Arabidopsis thaliana. Plant Cell Physiol 51(6):981–996. https://doi.org/10.1093/pcp/pcq057
Raghothama KG (1999) Phosphate acquisition. Annu Rev Plant Biol 50(1):665–693. https://doi.org/10.1146/annurev.arplant.50.1.665
Rook F, Hadingham SA, Li YH, Bevan MW (2006) Sugar and ABA response pathways and the control of gene expression. Plant Cell Environ 29:426–434. https://doi.org/10.1111/j.1365-3040.2005.01477.x
Secco D, Jabnoune M, Walker H, Shou HX, Wu P, Poirier Y et al (2013) Spatio-temporal transcript profiling of rice roots and shoots in response to phosphate starvation and recovery. Plant Cell 25:4285–4304. https://doi.org/10.1105/tpc.113.117325
Segami S, Tomoyama T, Sakamoto S, Gunji S, Fukuda M, Kinoshita S et al (2018) Vacuolar H+-pyrophosphatase and cytosolic soluble pyrophosphatases cooperatively regulate pyrophosphate levels in Arabidopsis thaliana. Plant Cell 30:1040–1061. https://doi.org/10.3389/fpls.2020.00655
Sowokinos JR, Spychalla JP, Desborough SL (1993) Pyrophosphorylases in Solanum tuberosum (IV. Purification, Tissue Localization, and Physicochemical Properties of UDP-Glucose Pyrophosphorylase). Plant Physiol 101(3):1073–1080. https://doi.org/10.1104/pp.101.3.1073
Spychalla JP, Scheffler BE, Sowokinos JR, Bevan MW (1994) Cloning, antisense RNA inhibition and the coordi nated expression of UDP-glucose pyrophosphorylase with starch biosynthetic genes in potato tubers. J Plant Physiol 144:444–453. https://doi.org/10.1016/S0176-1617(11)82121-8
Sun SB, Gu M, Cao Y, Huang XP, Zhang X, Ai PH et al (2012) A constitutive expressed phosphate transporter, OsPht1;1, modulates phosphate uptake and translocation in phosphate-replete rice. Plant Physiol 159:1571–1581. https://doi.org/10.1104/pp.112.196345
Woo MO, Ham TH, Ji HS, Choi MS, Jiang WZ, Chu SH et al (2008) Inactivation of the UGPase1 gene causes genic male sterility and endosperm chalkiness in rice (Oryza sativa L.). Plant J 54:190–204. https://doi.org/10.1111/j.1365-313X.2008.03405.x
Yin YB, Huang JL, Gu XG, Bar-Peled M, Xu Y (2011) Evolution of plant nucleotide-sugar interconversion enzymes. PLoS ONE 6(11):e27995. https://doi.org/10.1371/journal.pone.0027995
Yoon J, Cho LH, Tun W, Jeon JS, An G (2021) Sucrose signaling in higher plants. Plant Sci 302:110703. https://doi.org/10.1016/j.plantsci.2020.110703
Zhang N, Jiang J, Yang YL, Wang ZH (2015) Functional characterization of an invertase inhibitor gene involved in sucrose metabolism in tomato fruit. J Zhejiang Univ-Sci B 16(10):845–856. https://doi.org/10.1631/jzus.B1400319
Zhou J, Jiao FC, Wu ZC, Li YY, Wang XM, He XW et al (2008) OsPHR2 is involved in phosphate-starvation signaling and excessive phosphate accumulation in shoots of plants. Plant Physiol 146(4):1673–1686. https://doi.org/10.1104/pp.107.111443
Zou XH, Wei D, Wu PF, Zhang Y, Hu YN, Chen ST et al (2018) Strategies of organic acid production and exudation in response to low-phosphorus stress in Chinese fir genotypes differing in phosphorus-use efficiencies. Trees 32(3):897–912. https://doi.org/10.1007/s00468-018-1683-2
Acknowledgements
Thanks to Jiangsu Open University and Nanjing Agricultural University for providing laboratories and other experimental materials to support the experiment. The authors would like to express their gratitude to EditSprings (https://www.editsprings.cn) for the expert linguistic services provided.
Funding
This work was supported by Natural Science Research of Jiangsu Higher Education Institutions of China under Grant Agreement No22KJD210001.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, W., Meng, Q., Liu, W. et al. Overexpressing Ugp1 promotes phosphate uptake and accumulation in rice (Oryza sativa). Physiol Mol Biol Plants 29, 1409–1421 (2023). https://doi.org/10.1007/s12298-023-01368-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-023-01368-8