Abstract
The present study involved two pot experiments to investigate the response of mung bean to the individual or combined SO42− and selenate application under drought stress. A marked increment in biomass and NPK accumulation was recorded in mung bean seedlings fertilized with various SO42− sources, except for CuSO4. Compared to other SO42− fertilizers, ZnSO4 application resulted in the highest increase in growth attributes and shoot nutrient content. Further, the combined S and Se application (S + Se) significantly enhanced relative water content (16%), SPAD value (72%), photosynthetic rate (80%) and activities of catalase (79%), guaiacol peroxidase (53%) and superoxide dismutase (58%) in the leaves of water-stressed mung bean plants. Consequently, the grain yield of mung bean was markedly increased by 105% under water stress conditions. Furthermore, S + Se application considerably increased the concentrations of P (47%), K (75%), S (80%), Zn (160%), and Fe (15%) in mung bean seeds under drought stress conditions. These findings indicate that S + Se application potentially increases the nutritional quality of grain legumes by stimulating photosynthetic apparatus and antioxidative machinery under water deficit conditions. Our results could provide the basis for further experiments on cross-talk between S and Se regulatory pathways to improve the nutritional quality of food crops.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate change has increased the vulnerability of cropping systems, which may trigger a loss of livelihoods of poor communities in arid and semi-arid regions of the world (Leng and Hall 2019). The imbalance in the temporal and spatial distribution of rainfall and the non-availability of the required amount of water during critical growth stages would seriously limit the productivity of major food crops in near future (Troy et al. 2015). The non-availability of water severely hampers crop production in developing and arid regions of the world where people are solely dependent on rainwater for their survival. Detrimental effects of drought are likely to aggravate the world’s limiting available fresh water supply to fulfill the food demand for the ever-increasing population (Leng 2017). In this regard, it is imperative to recognize the importance of climate-resilient crops such as legumes and promote their value throughout the food systems.
Legumes are an integral part of a healthy and balanced human diet and play important role in preventing many acute diseases. Moreover, due to their atmospheric nitrogen (N) fixing ability, they increase soil C and N, reduce soil erosion and help to control soil pathogens (Rubiales and Mikic 2015). Mung bean (Vigna radiata (L.) R. Wilczek) is an important food legume that is grown primarily for dry seeds (Kudre et al. 2013). Although, mung bean is considered tolerant to limited water supply, low water availability at reproductive and grain filling stages significantly reduces its yield and quality (Gaur et al. 2012). Drought-induced changes in mung bean include reduced carbon fixation, repressed flowering time, increased pollen sterility, fewer pods, and poor seed set (Nadeem et al. 2019). Legume crops like mung bean are commonly cultivated in rainfed regions; hence, development of sustainable mitigation approaches that could easily integrate with farming practices of dry areas is essential to ensure high yield under harsh environment (Raina et al. 2016). Application of mineral nutrients actively involved in stress tolerance and defense mechanisms could be used as a viable option to alleviate the damaging effects of environmental stresses in legumes (Afzal et al. 2015).
Sulfur (S) is an important element that plays a crucial role to mitigate abiotic stresses in crop plants. Increased sulfate (SO42−) demand during metabolic adaptation to drought reflects its importance in the regulation of plant defense machinery (Usmani et al. 2020). Drought stress increases the concentration of SO42− compared to other ions like phosphate or nitrate, providing evidence that sulfate demand increases under limited water conditions (Ernst et al. 2010). SO42− interacts with abscisic acid (ABA) and acts as a chemical signal to initiate stomatal closure in leaves under water deficit conditions. Metabolism of S results in the formation of several S-containing defense compounds such as methionine, cysteine, glutathione, and phytochelatins that are involved in plant survival during various abiotic stresses including drought (Honsel et al. 2012). The interplay of S with phytohormones helps to regulate crucial metabolic processes in plants (Noctor et al. 2012). Deficiency of S may result in impaired growth, loss in plant production, and susceptibility to environmental stresses due to reduced protein synthesis, chlorophyll degradation and decreased photosynthetic rate (Fatma et al. 2016).
Akin to S, selenium (Se) is an important micronutrient considered essential for animals and required in very minute quantities by plants. Both Se and S share similar chemical properties and are taken up by plants through sulfate transporters and assimilated by S assimilating pathway (Sors et al. 2005). In most countries, the major dietary source of Se is plant food (Rayman 2008). Se deficiency in the human diet causes abnormalities in thyroid function alters bone metabolism, and leads to retarded growth (Reeves and Hoffman 2009). In Se deficient areas, exogenous application with Se or Se-enriched compounds can be used as an efficient, cost-effective and viable approach to enrich plants with Se (Sabatino et al. 2019). Moreover, Se application stimulates growth, improves physiological processes (Bocchini et al. 2018), and enhances tolerance against various environmental stresses (Banerjee and Roychoudhury 2019). It is considered as a constituent of selenoenzymes (thioredoxin reductases, glutathione peroxidase, and proteins), which are vital to maintaining cell redox potential (Rayman 2008). Se increases plant growth (Nawaz et al. 2013), carbohydrate accumulation (Kaur et al. 2018), and delays senescence in crop plants (Hajiboland et al. 2019). The positive effects of Se to mitigate biotic and abiotic stresses including UV-B radiation (Golob et al. 2017), cadmium toxicity (Shahid et al. 2019), fungal infections (Kornaś et al. 2019), and drought (Nawaz et al. 2015a) are well documented.
To date, only few studies have been conducted to investigate the yield changes in leguminous crops like mung bean by Se supply under drought stress. This study partially fills the gap by determining the effects of Se application and its interrelation with S in mung bean exposed to water deficit conditions. The study aimed to i) evaluate the effects of Se or S nutrition on physiological mechanisms of mung bean under drought stress and ii) assess whether combined application of Se and S is more effective than individual treatment to improve yield and mineral content of mung bean under water deficit conditions.
Materials and Methods
Experimental conditions and material
The experiments were conducted in two phases: initially, the locally cultivated mung bean cultivars were screened for their response to various S fertilizers in the first experiment. Later in the second experiment, the most responsive mung bean cultivar was evaluated for its response to individual or combined S and Se doses under normal and water-deficient conditions. The experiments were performed in a green house (C-block, MNS-University of Agriculture, Multan, Pakistan) with ambient temperature (average day/night: 36/23 °C) and light (photoperiod: 14/10 h) conditions during the growth period. A manually controlled moveable polythene sheet was installed to protect the plants from rainwater. The experimental design was completely randomized design (CRD) with the factorial arrangement and three replications (03 plants per replication/pot were maintained till final harvest). The pot experiment-I comprised of 45 pots, whereas 36 pots were used in the pot-experiment-II. The pots were filled with sun-dried, grounded, and thoroughly mixed clayey-loamy soil collected from the farm area of the University located at B-block. The physicochemical characteristics of the soil were determined following the reports of Jackson and Barak (2005) and given in Table 1.
Pot experiment-I
The first experiment was conducted with the objective to evaluate the response of mung bean cultivars to various S-sources. The experiment was initiated on May 06, 2018, for four weeks. Seeds of three local mung bean cultivars viz. NM-2006, NM-2011, and AZRI-2006 were obtained from Punjab Seed Corporation, Faisalabad (Pakistan). Initially, six healthy and physically pure seeds were sown in pots with 15 L capacity (height 25 cm and diameter 30 cm), and each filled with 12 kg clayey-loamy soil. After the establishment of seedlings, thinning was done to maintain three healthy plants per pot. Before sowing, recommended doses of fertilizers such as urea (25 kg ha−1, 150 mg pot−1) and diammonium phosphate (50 kg ha−1, 300 mg pot−1), and potassium hydroxide (50 kg ha−1, 300 mg pot−1) were added as nutrient solutions to meet the nutritional requirement of the plants in each pot. To ensure that each pot received an equal amount of S (20 kg ha−1, 120 mg pot−1), different fertilizer treatments of zinc sulfate (ZnSO4, 1090 mg pot−1), iron sulfate (FeSO4, 571 mg pot−1), potassium sulfate (K2SO4, 667 mg pot−1), and copper sulfate (CuSO4, 923 mg pot−1) were added through fertigation in each pot. After four weeks, the seedlings were harvested to determine the effects of S fertilization on biomass accumulation and nutrient uptake in different mung bean cultivars. The seedlings were washed thoroughly to avoid any sand particles and the samples were packed in plastic bags and tagged according to the treatments.
Pot-experiment II
In this experiment, the role of S and/or Se nutrition to improve the growth and yield of mung bean under drought stress was investigated. The best performing mung bean cultivar i.e. AZRI-2006, selected from pot-experiment-I, was evaluated for its response to individual or combined S and Se nutrition. The S-fertilizer (ZnSO4, 1090 mg pot−1) was selected based on results of pot-experiment I and was evaluated alone or in combination with two Se doses i.e. Se1 (2 μmol Se L−1) and Se2 (4 μmol Se L−1) using sodium selenate (Na2SeO4) as a source of Se. The experiment was conducted during the growth period of 2018 (June 14 to September 20). Physically pure and healthy seeds of selected mung bean cultivar (AZRI-2006) were sown in brick pots, as described in pot-experiment-I. The nutritional requirements in the form of N, P, and K along with ZnSO4 were fulfilled at the start of the experiment (two days before sowing) as described earlier, whereas Se treatments as foliar spray were done one week after the onset of drought.
Drought stress and foliar Se treatment
All pots were watered regularly considering the moisture requirements calculated using the method reported by Giriappa (1988). The seedlings were allowed to establish under normal conditions for the first two weeks and then drought stress was initiated by withholding water supply for the next 15 days to one set of pots (drought stress) compared to normal plants watered at regular intervals. Foliar spray of Se was done one week after the initiation of drought using compression sprayer as described by Nawaz et al. (2015a) and repeated the next week on the same day. The spraying was done manually in the early hours of the morning (between 8:00 and 9:00 a.m.) and each treatment solution was added with Tween-20 (0.1%) to enhance the adhesion of Se to foliage. Data regarding gas exchange characteristics was recorded two days after the second spray, whereas the leaf samples for physiological and biochemical analysis were collected the very next day. The plants were grown till maturity and harvested manually at physiological maturity when about more than 80% of pods turned yellow.
Morphological attributes and shoot NPK content
The seedlings were manually uprooted to collect data regarding biomass accumulation. The soil around the seedlings was dug deep and seedlings were removed with extreme care to minimize damage to the roots. A metering rod was used for the measurement of the root length (RL) of each seedling. Observed data from selected plants were recorded in cm (centimeters) from the basal end of the root to the bottom of the shoot and the mean of three readings was calculated. The shoot length (SL) of above-ground part of each seedling was calculated in centimeters (cm) using a meter rod. The seedlings were then weighed immediately to record shoot fresh weight (SFW) in grams (g) using a digital balance. The shoots were then placed in an oven at 65 °C for 72 h to record shoot dry weight (SDW).
The oven-dried shoot samples (above ground material) were grounded and later these finely ground shoot samples (0.5 g) were acid digested using 5 ml HNO3 and 2 ml H2O2 at 350 °C for three hours in digestion block. This was done until the solution becomes colorless. After digestion, the solution was cooled to room temperature and distilled water was added to make the final volume up to 50 ml. The shoot N content was determined by Kjeldahl method (AOAC 1984), whereas the Vanadium molybdate yellow calorimetric method (Chen et al. 1956), and Flame photometry (Karpiuk et al. 2016) were employed to estimate P and K contents in shoots, respectively.
Estimation of leaf water status and SPAD value
The leaf water status of normal and drought-stressed mung bean plants was estimated as leaf relative water content (RWC) using leaf samples (0.5 g) from the uppermost fully expanded leaf (Schonfeld et al. 1988). The leaves were weighed immediately (FW), re-hydrated in distilled water for 24 h in the refrigerator (4 °C) to record turgid weight (TW), and later dried in an oven at 70 °C until constant weight (DW) was obtained. Leaf RWC was calculated as 100 x (FW-TW)/(FW-DW).
Randomly selected top five fully expanded leaves were selected in each pot to measure leaf total chlorophyll content (Chl) using SPAD meter (SPAD-502 plus, Konica-Minolta, Beijing, China). The readings were used to calculate the average SPAD value for each treatment.
Measurement of leaf gas exchange characteristics
The leaf gas exchange characteristics viz. photosynthetic rate (A), stomatal conductance (gs), sub-stomatal conductance (Ci), and transpiration rate (E) were recorded from the second fully expanded young mature leaf from the top using portable photosynthesis system CIRAS-3 (PP Systems, Amesbury, U.S.A.). These observations were made in the morning between 8:00 and 10:00 with the adjustments as reported in Shehzad et al. (2020).
Antioxidative enzymes assay
Fresh leaf samples (0.5 g) were homogenized in an ice-cold mortar and pestle (1:5 w/v) using a mixture of sodium phosphate buffer (50 mM, pH 7.0), polyvinylpyrrolidone (1%), EDTA (1 mM), and NaCl (1%). The crude extract was centrifuged at 20,000xg for 15 min and the supernatant, denoted as enzyme extract (EE), was used to determine the antioxidant enzyme activity.
Catalase (CAT) activity was determined following the procedure of Aebi (1984). Briefly, 200 μL EE was added to 1.8 mL RM (reaction mixture) prepared by mixing K-P-buffer (50 mM, pH 7.0) and H2O2 (30 mM). The degradation of H2O2 was recorded as a reduction in optical density at 240 nm.
The method published by Urbanek et al. (1991) was followed to determine the leaf guaiacol peroxidase (GPX) activity. Initially, 25 μL EE was added to 2 ml solution prepared by mixing K-P-buffer (50 mM, pH 6.8), H2O2 (20 mM), and guaiacol (20 mM). After incubation at room temperature for 10 min, the reaction was stopped by adding 0.5 mL H2SO4 (5% v/v) and the absorbance was noted at 480 nm.
The enzyme activity of superoxide dismutase (SOD) was assayed by adding 50 μL EE in RM containing L-methionine (13 mM), NBT or nitro blue tetrazolium chloride (75 μM), riboflavin (2 μM)) and EDTA (100 μM) in K-P-buffer (50 mM, pH 7.8). The reaction was initiated under the illumination of a 30 W-fluorescent lamp for 5 min, resulting in the formation of blue formazan measured as an increase in absorbance at 560 nm using RM (kept in the dark) as a blank (Van Rossum et al. 1997).
Determination of yield components seed mineral content
All plants were harvested manually at physiological maturity. The number of seeds in each pod was collected and then weighed to obtain 100-grain weight (GW) for each treatment. The biological yield (BY) was determined by weighing the total plants in each pot and then the average was calculated. Similarly, grain yield (GY) was determined by weighing the total number of grains produced by plants per pot, and later average number of grains per plant was calculated.
Finely ground seeds (0.2 g) of each treatment were digested by HNO3 to determine the concentrations of mineral nutrients. The potassium (K), zinc (Zn), iron (Fe), and manganese (Mn) concentrations were measured following the method of Karpiuk et al. (2016) while the S content was determined according to Chandra and Pandey (2016) using atomic absorption spectrophotometry. Also, the seed N and P concentrations were measured on a UV–visible spectrophotometer (Hitachi-220, Hitachi Ltd, Tokyo, Japan) using Kjeldahl method (AOAC 1984) and molybdenum reaction solution method (Chen et al. 1956), respectively.
Statistical analyses
Fisher’s ANOVA (analysis of variance) technique was employed to statistically analyze all recorded data using Statistix (Analytical Software, FL, USA, Version 8.1). The treatment means were compared using Tukey’s posthoc HSD test at a 95% confidence level (P < 0.05).
Results
Biomass attributes
All biomass traits exhibited highly considerable (P ≤ 0.01) variation among mung bean cultivars (Suppl. Table 1). The cultivar AZRI-2006 maintained significantly higher SL (12.64 cm), RL (12.12 cm), SFW (0.72 g), and SDW (0.27 g) compared to NM-2006 and NM-2011 (Fig. 1). Among different SO42− fertilizers, ZnSO4 application resulted in the highest SL (37%), RL (51%), SFW (26%), and SDW (25%) in AZRI-2006 with respect to control. Application of FeSO4 and K2SO4 also improved the biomass of AZRI-2006 seedlings only, whereas CuSO4 markedly reduced these attributes in all mung bean cultivars (Fig. 1).
Effect of different S-sources viz. no S supply (control), ZnSO4, FeSO4, K2SO4 and CuSO4 on a shoot length, b root length c shoot fresh weight and d shoot dry weight of three mung bean cultivars (NM-2006, NM-2011 and AZRI-2006). Treatment means that are significantly different are represented by different letters, after applying post-hoc Tukey’s HSD test
Shoot NPK content
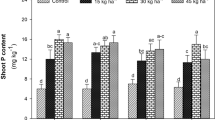
Among mung bean cultivars, a marked variation for shoot NPK content was only noted for K accumulation as AZRI-2006 exhibited 13 and 12% higher K content than NM-2006 and NM-2011, respectively (Fig. 2c). However, different S fertilizers showed a significant effect on N, P, and K accumulation in shoots of mung bean cultivars (P ≤ 0.01; Suppl. Table 1). Mung bean cultivars treated with ZnSO4 exhibited 25 and 9% higher shoot N content than FeSO4 and K2SO4, respectively (Fig. 2a). A similar trend was noted for shoot P content with a maximum increase (25%) recorded in shoots of seedlings fertigated with ZnSO4 compared to control (no S fertilizer). Likewise, the K2SO4 application also improved shoot P content by 16%, whereas no significant effect of FeSO4 and CuSO4 was observed on this variable (Fig. 2b). The shoot K content was increased by 30 and 21% in seedlings treated with ZnSO4 and K2SO4, respectively however, CuSO4 application did not affect K accumulation compared to control (Fig. 2c).
Effect of different S-sources viz. no S supply (control), ZnSO4, FeSO4, K2SO4 and CuSO4 on shoot a nitrogen, b phosphorous and c potassium content of three mung bean cultivars (NM-2006, NM-2011 and AZRI-2006). Treatment means that are significantly different are represented by different letters, after applying post-hoc Tukey’s HSD test
Leaf RWC and SPAD value
Drought stress considerably decreased (P ≤ 0.01) the leaf RWC of mung bean plants (Suppl. Table 2). Under well-watered and drought conditions, the combined application of S and Se (S + Se2) resulted in maximum leaf RWC and improved it by 10 and 16% in normal and water-stressed plants, respectively compared to control. Individual application of ZnSO4 (S) and low (Se1) or high Se dose (Se2) also improved leaf RWC by 9 and 12%, respectively under drought stress conditions (Fig. 3a).
The individual and combined effect of S (ZnSO4) and Se (Na2SeO4) on a leaf relative water content and b total chlorophyll content of mung bean plants under well-watered and drought stress conditions. S1 = 120 mg S pot−1, Se1 = 2 μmol Se L−1, Se2 = 4 μmol Se L−1, S + Se1 = 120 mg S pot−1 + 2 μmol Se L−1 and S + Se2 = 120 mg S pot−1 + 4 μmol Se L−1. The significant differences among treatment means are represented by different letters, after applying post-hoc Tukey’s HSD test
The exposure to drought showed a marked reduction (32%) in leaf Chl content (measured as a SPAD value) of mung bean plants (P ≤ 0.01, Suppl. Table 2). Under well-watered conditions, no significant effect of individual or combined S and Se doses was observed on this variable, whereas S + Se1 markedly enhanced leaf Chl (72%) under drought stress conditions (Fig. 3b). The leaf Chl were also increased by individual Se application as low (Se1) or high Se dose (Se2) improved SPAD value by 38 and 34%, respectively while ZnSO4 supply alone (S) did not affect leaf Chl compared to control under water deficit conditions (Fig. 3b).
Gas exchange
A significant variation (P ≤ 0.01) existed among individual or combined treatment of S and Se for leaf gas exchange attributes under well-watered and drought stress conditions (Suppl. Table 2). Under well-watered conditions, the highest increase (32%) in leaf A was observed in plants treated with S in combination with 2 μmol Se L−1 i.e. S + Se1 however, it resulted in a much higher increase (80%) in leaf A statistically related to S + Se2 (120 mg S pot−1 + 4 μmol Se L−1) compared to control (no S and/or Se application) under drought stress (Table 2). In the plants exposed to drought stress, leaf gs and Ci were increased by 87 and 92%, respectively by S + Se2 application, in contrast to 62 and 23% increase in these variables compared to control under normal conditions (Table 2). Similarly, the highest leaf E was also maintained by the plants treated with combined S + Se2 application (88%) statistically at par with S + Se1 (85%) with respect to control under drought stress conditions (Table 2).
Enzyme antioxidants
Drought stress markedly enhanced the activities of enzyme antioxidants viz. CAT, GPX, and SOD in the leaves of mung bean plants, irrespective of S and Se treatments (Suppl. Table 2). The highest increase in activity of antioxidative enzymes was recorded in the combination of S and Se treatments i.e. S + Se1 and S + Se2 (Fig. 4). Mung bean plants treated with S + Se2 exhibited the highest CAT (79%) and GPX (53%) activity statistically related to S + Se1 treatment with respect to control under drought stress. Among individual treatments, foliar spray of high Se dose (Se2) also stimulated CAT (28%) and GPX (37%) activities, whereas low Se dose (Se1) or S application enhanced CAT activity by 17 and 26% (Fig. 4a) and GPX activity by 19% (Fig. 4b) compared to control under water deficit conditions. The highest increase in SOD activity (81%) was also recorded in water-stressed mung bean plants applied with combined S + Se2 treatment (Fig. 4c). No significant variation for SOD activity was recorded between S + Se1 and Se2 treatments that stimulated enzyme activity by 58%, whereas Se1 and S did not considerably affect SOD activity with respect to control under water deficit conditions.
The individual and combined effect of S (ZnSO4) and Se (Na2SeO4) on enzyme activity of a catalase b guaiacol peroxidase and c superoxide dismutase in leaves of mung bean plants under well-watered and drought stress conditions. S1 = 120 mg S pot−1, Se1 = 2 μmol Se L−1, Se2 = 4 μmol Se L−1, S + Se1 = 120 mg S pot−1 + 2 μmol Se L−1 and S + Se2 = 120 mg S pot−1 + 4 μmol Se L−1. The significant differences among treatment means are represented by different letters, after applying post-hoc Tukey’s HSD test
Yield attributes
Drought stress, in relation to well-watered conditions, caused a marked reduction in mung bean yield (P ≤ 0.01, Suppl. Table 3). Mung bean plants treated with S and Se in combination (S + Se1, S + Se2) showed marked improvement in GW, GY and BY under both well-watered and drought stress conditions. In comparison to no S or Se application (control), combined S + Se2 application resulted in the highest increase in GW (11%), GY (105%), and BY (75%) followed by S + Se1 treatment that improved GW, GY, and BY by 7, 68 and 48%, respectively under water deficit conditions, whereas no significant differences were obtained when S or Se was applied as individual treatments (Fig. 5).
The individual and combined effect of S (ZnSO4) and Se (Na2SeO4) on a 100-grain weight b grain yield and c biological yield of mung bean plants under well-watered and drought stress conditions. S1 = 120 mg S pot−1, Se1 = 2 μmol Se L−1, Se2 = 4 μmol Se L−1, S + Se1 = 120 mg S pot−1 + 2 μmol Se L−1 and S + Se2 = 120 mg S pot−1 + 4 μmol Se L−1. The significant differences among treatment means are represented by different letters, after applying post-hoc Tukey’s HSD test
Seed mineral content
Under drought stress, the nutrient content (N, P, K, Zn, S, and Fe) in seeds of mung bean plants was considerably decreased (Suppl. Table 3). Particularly, seed K content was decreased by 48%, whereas P, Zn, and S concentrations in seeds were reduced by 65, 68 and 96%, respectively compared to seeds of plants grown under well-watered conditions (Table 3). Seeds from mung bean plants grown with S, Se or S + Se showed considerable enhancement in mineral content. As compared to no S or Se supply (control), the highest increase in seed concentrations of P (47%) and K (75%) was recorded by S + Se1 (Table 3), whereas S + Se2 resulted in the maximum seed N content (12%) closely followed by Se2 (11%) and S + Se1 (10%) under drought stress conditions (Table 3). Although differences in S and Zn seed concentrations between the individual or combined S or Se treatments were not significant under well-watered conditions, a marked enhancement in seed S (80%) and Zn (160%) content was recorded by S + Se1 treatment compared to control under water deficit conditions (Table 3). Similarly, S + Se2 and Se2 improved Zn seed concentrations by 125 and 67%, respectively while no application (control) gave the lowest values for Zn seed concentrations followed by individual S or low Se supply (Se1) treatments in water-stressed mung bean plants (Table 3). Under well-watered conditions, seed Fe and Mn concentrations were significantly increased by 15 and 26%, respectively in plants treated with combined S and Se applications i.e. S + Se1 and S + Se2 compared to control. A similar trend was noted in water-stressed plants as S + Se2 resulted in the highest (15%) seed Fe content (Table 3), however, a much higher increase of 37% was noted for Mn concentration by this treatment compared to control (Table 3). In contrast, individual S and Se doses were found statistically similar to control regarding seed Fe and Mn content.
Discussion
Most agricultural soils lack S and contain less than 10% of the total soil S in the plant available form i.e. SO4 (Urton et al. 2018). Consequently, the organic S sources (soil organic matter and crop residues) are primary SO4 sources for plants during the growth season (Havlin et al. 2014). The increase in biomass of mung bean cultivars in response to different S fertilizers compared to control (no S supply) is consistent with the availability of low SO4 levels in soil used for these experiments. A marked improvement in biomass was noted by S fertilizer amendments, which may be due to the positive effects of S on cell division resulting in leaf expansion and light absorption that ultimately improved the vegetative growth (Garg et al. 2006). A highly significant increase in SL was noted in plants supplemented with various SO4 fertilizers except CuSO4 (Fig. 1a). Similarly, a considerable increase in RL and dry matter content was also observed in mung bean plants treated with S fertilizers (Fig. 1). Availability of SO4 in growth medium is positively correlated to indole-3-acetic acid (IAA) levels in root meristem that promotes primary root elongation to facilitate the uptake of nutrients and moisture (Zhao et al. 2014). A positive effect of S fertilizers on the dry matter of mung bean plants might be due to increased carbohydrate utilization for protein synthesis in the presence of S (Shekhawat and Shivay 2012). These results are in line with studies on wheat (Salvagiotti and Miralles 2008) and barley (De Bona et al. 2011) grown on SO4 deficient soils. Among various S fertilizers, the maximum biomass was attained in plants fertilized with ZnSO4. It might be attributed to regulatory functions of both Zn2+ and SO42− to stimulate the activity of enzymes such as aldolases, hydrogenases, nucleic acid polymerases. Moreover, they also act as a cofactor to regulate protein synthesis thereby contributing to increased biomass in plants (Rossi et al. 2018). The possible explanation for the decrease in biomass by CuSO4 application may be the presence of Cu2+ that alters root morphology to decrease xylem loading of water and nutrients in plants (Chen et al. 2015).
The metabolism of N in plants is strongly affected by the S status of the plant (Anjum et al. 2012). It was observed that NPK uptake was considerably higher in AZRI-2006 compared to NM-2006 and NM-2011 (Fig. 2). These results suggest that AZRI-2006 has a high capacity to uptake NPK and can respond better to SO42− availability. Our results are in agreement with reports of Lee et al. (2015) who observed significant variation among Brassica species in their response to S nutrition. Among S-fertilizers, ZnSO4 and FeSO4 markedly improved N uptake in mung bean plants (Fig. 2a). The increased N content by ZnSO4 might be related to increased activity of SO42− transporters in the root with Zn2+ exposure resulting in enhanced NO3− absorption and translocation in leaves (Stuiver et al. 2014). Plants have well-developed S and N pathways, which are functionally convergent as the availability of one nutrient regulates the other (Kaur et al. 2013). A marked increment in P uptake was observed in plants treated with S-fertilizers. Taalab et al. (2008) reported that combined application of different P sources with S results in better uptake of macronutrients such as N, P, and K compared to P alone. Likewise, S-application enhanced K content in the shoot, which might be associated with the fact that S and K act as counter-ions (Bäucker et al. 2003). Chinese cabbage plants exposed to S deficiency were observed to accumulate fewer K+ in the shoot by Reich et al. (2016). Increased NPK accumulation in mung bean leaves by FeSO4 application provides further evidence regarding the cooperative effect of Fe-S clusters on plant metabolism (Lu 2018). In contrast, Cu2+ exposure in the root zone markedly decreases the uptake and distribution of SO42− (Shahbaz et al. 2014) that also reduces NPK uptake due to the limited incorporation of proteins in leaves.
Mung bean is considered a drought-tolerant crop, however; water deficiency at critical growth stages may result in significant yield losses (Singh and Singh 2011). Application of S has been observed as increasing the resistance of plants under drought stress as it helps to regulate metabolic processes and is the main constituent of metabolites involved in protein synthesis under water-limited conditions (Vazin 2012). Foliar treatment of mung bean plants with Se in combination with S (S + Se) considerably improved RWC (Fig. 2a), which signifies synergistic relation of these nutrients in the accumulation of solutes to sustain water status under drought (Hartikainen 2005). Se increases water retention in the plant tissues through improved uptake of water by the root system (Nawaz et al. 2015a). The regulation of stomatal apparatus with S + Se supply (as observed in the present study, Table 2) might be responsible for improved leaf RWC under water stress conditions (Usmani et al. 2020). The hydraulic conductance of plant species is a major function for increased water status under stress conditions (Schultz 2003) hence, an increase in RWC of plants treated with S + Se indicates the positive role of these nutrients in increasing water uptake by roots without decreasing E under water deficit conditions. S availability helps to maintain osmotic potential by restricting the cellular accumulation of ions and solutes (Lee et al. 2015). Similarly, Se helps to maintain membrane integrity (Chauhan et al. 2019) or reduces oxidative damage (Shekari et al. 2019). Photosynthetic pigments like chlorophyll indicate the photosynthetic capacity of plants (Chutipaijit et al. 2011). It is well reported that exogenous S or Se supply prevents the damage to chloroplasts and increases the pigments under drought stress conditions (Usmani et al. 2020). A marked increase in SPAD value (an indicator of total leaf Chl) was noted with S + Se supply which might be associated with sulphurs’ role in the maintenance of chloroplast targeted proteins (cysteine and methionine) as well as Se mediated scavenging of ROS through increased activity of antioxidants (also observed in the present study) to inhibit lipid peroxidation that leads to chlorophyll destruction (Habibi 2013).
As expected, low water availability markedly reduced A, E, gs, and Ci in leaves of mung bean plants. The reduction in A result from decreased gs that restricts CO2 assimilation and uptake of nutrients from the soil due to limited E. In this study, the application of S + Se considerably improved A (Table 2) suggesting these nutrients work synergistically to prevent Rubisco deactivation, chlorophyll degradation, and stomatal closure. This might also be attributed to the fact that both S and Se positively influence turgor maintenance, thereby increasing tolerance in a water-limited environment. S deficiency has been reported to severely restrict A in Brassica napus (Muneer et al. 2014), Brassica juncea (Fatma et al. 2014), and Hordeum vulgare (Astolfi et al. 2012) indicating that S is crucial to maintain A in plants. S is a vital constituent of ferredoxin and lipids found in the chloroplast. Moreover, sulfur bonds (-S–S-) are very crucial for maintaining protein structure and disulfide groups are constituents of active sites of many enzymes (Speiser et al. 2018). Application of Se and S increased the A, E, and gs, however, maximum activity was observed at combined higher doses of Se and S. This may be due to the synergistic effects of S and Se as both these nutrients share a similar assimilation pathway (Sors et al. 2005). Se-mediated increase in A, E, and gs has also been reported by Nawaz et al. (2015a) in wheat and Proietti et al. (2013) in olive plants. Moreover, Djanaguiraman et al. (2010) showed that Se-spray elevated A in sorghum that might be related to Se-mediated low ROS levels and enhanced antioxidant activities.
A marked increment in enzyme activities of antioxidants was recorded by the application of S and Se in water-stressed mung bean plants, which could be attributed to the protective role of these enzymes to detoxify the elevated H2O2 into water and oxygen (Anjum et al. 2012). Enhanced enzyme activity of antioxidants in mung bean supplemented with exogenous Se has also been reported earlier (Malik et al. 2012; Alam et al. 2019). A well-organized antioxidant system plays a critical role to scavenge ROS under environmental stress conditions (Karl et al. 2009). The observed increase in SOD activity of mung bean plants by combined application of S (120 mg pot−1) and Se (4 μmol L−1) under drought stress (Fig. 4c) provides further evidence about the protective characteristics of S and Se to attenuate damaging effects of ROS and regulate the osmotic status of plants (Hajiboland et al. 2015). Plants produce SOD as a primary defense enzyme to scavenge ROS produced under drought (Hussain et al. 2016). Turkan et al. (2005) reported higher activity of CAT in drought-tolerant varieties of bean cultivars compared to sensitive ones. Plants supplemented with S + Se showed higher GPX and CAT activity and increased tolerance to the water-limited conditions. Increased GPX activity by Se supplementation corresponds to the reports of Yao et al. (2012). It may be inferred that high GPX activity promotes lignin biosynthesis that contributes to enhanced cell wall rigidity and increased uptake of mineral nutrients, thereby regulating the defense mechanism of plants to scavenge H2O2 (Gill and Tuteja 2010; Majeed et al. 2020).
Drought-induced significant reduction in mung bean yield was recorded in the present study (Fig. 5). The reduction in yield mainly results from a decreased translocation of nutrients due to the short duration of seed filling induced by drought stress (Nair et al. 2019). Moreover, water deficiency at the flowering stage causes flower and pod abortion resulting in small-sized seeds (Fang et al. 2010). In the present study, a significant increase in GW was recorded with combined S and Se application. S-mediated enhancement in yield has been reported in crops such as chickpea (Abd Allah et al. 2015), maize (Usmani et al. 2020), wheat (Tabak et al. 2019), and mung bean (Sachan et al. 2019). It has been suggested that S supply in combination with Se increases nutrient uptake particularly N and its translocation for the incorporation of proteins in seeds (Becana et al. 2018). Moreover, S + Se regulated enhanced photosynthetic activity also positively influenced the GW. A marked increase in BY was recorded by combined S + Se application (Fig. 5b). It is more likely the consequence of efficient translocation of assimilates resulting in increased dry matter thereby increasing BY under water-deficient conditions. The increase in GY by S + Se could be ascribed to improved water status (Fig. 2a) and gas exchange characteristics (Table 2), thereby improving GY under water deficit conditions.
The concentrations of minerals in grains are regulated by direct uptake from soil and/or remobilization of nutrient reserves in tissues during grain filling (Kutman et al. 2011). Exposure to drought impairs root architecture resulting in reduced nutrient uptake and changes in quantity of nutrients accumulated during seed filling (Sehgal et al. 2018). In this study, fewer negative effects of drought were noted on the nutrient content of mung bean seeds obtained from plants treated with the combined application of S and Se. Limited water availability restricts SO42− uptake and its translocation to leaves (Usmani et al. 2020). Moreover, it also reduces nitrogen use efficiency (NUE) due to decreased NO3− absorption under water deficit conditions (Lee et al. 2009). Drought stress decreases protein incorporation in leaves due to low S demand that significantly limits SO42− uptake in water-stressed plants (Anderson and Fitzgerald 2003). Previous studies showed marked increment in S accumulation by Se application in lettuce (Ramos et al. 2011) Arabidopsis (White et al. 2004) and Kale (Lefsrud et al. 2006). Similarly in the present study, NPK content in seeds was significantly enhanced by S + Se application in water-stressed plants (Table 3). Foliar Se spray not only increased N, P, and K concentrations but also enhanced Zn, S, Fe, and Mn content in mung bean seeds. Similarly, Reis et al. (2018) reported positive interaction between Se and N that significantly increased the quality of rice seeds. In contrast, a negative correlation was recorded between Se and N in barley (Ilbas et al. 2012), which may be due to application of high Se doses resulting in Se toxicity. Similar to our observations, Eich-Greatorex et al. (2010) observed high P uptake by Se application suggesting that Se interactions with phosphate are largely dependent on the source of Se as selenite uptake is not efficient in P-enriched medium (Dinh et al. 2019). Contrary to our earlier report about no significant effect of Se on K content of wheat seeds (Nawaz et al. 2015b), an increased K content was recorded in mung bean seeds with S + Se treatment. Likewise, S content were also improved by combined S and Se application supporting the observations of Guerrero et al. (2014) who observed that selenate promotes translocation of S and noted increased shoot S content in wheat. There was an increase in seed Zn content by S + Se application, which may likely be the consequence of the application of ZnSO4 as a source of S. However, Se application combined with S resulted in high Zn content compared to individual treatment of S or Se (Table 3). These observations correspond to the reports of de Figueiredo (2017) who observed that common bean plants treated with Se or Zn maintained a constant level of these minerals during seed development. An increased seed Fe and Mn content by S + Se application could partly be ascribed to positive interactions of Fe with S and Se that favors the formation of Fe-S clusters in cells involved in the photosynthetic activity (Nazar et al. 2011) thereby, alleviating negative effects of drought stress on uptake and translocation of mineral nutrients.
Conclusion
In conclusion, the combined application of S and Se (S + Se) was observed to confer drought tolerance in mung bean, by alleviating the damage caused by drought stress and stimulating the physiological processes such as increased water status, chlorophyll content and photosynthetic activity. Moreover, the increased activity of antioxidative enzymes by S + Se application mitigated water deficit stress and improved yield and mineral status of mung bean under drought. These results could benefit farmers to obtain high nutrient concentrations in mung bean seeds with a limited supply of water. Besides, these results may also be of great interest to researchers including plant breeders to identify candidate genes regulated by both S and Se, which play an important role in improving the drought tolerance and controlling mineral accumulation in seeds.
References
Aebi H (1984) Catalase in vitro. Meth Enzymol 105:121–126
Afzal F, Khan T, Khan A, Khan S, Raza H, Ihsan A, Ahanger MA, Kazi AG (2015) Nutrient deficiencies under stress in legumes: an overview. In: Azooz MM, Ahmad P (eds) Legumes under environmental stress: yield, improvement and adaptations. Wiley, New York, pp 53–65
Alam MZ, McGee R, Hoque MA, Ahammed GJ, Carpenter-Boggs L (2019) Effect of Arbuscular Mycorrhizal Fungi, Selenium and Biochar on Photosynthetic Pigments and Antioxidant Enzyme Activity Under Arsenic Stress in Mung Bean (Vigna radiata). Front Physiol 10
Allah MMSA, Eldin MM, Selim SM (2015) Effect of nitrogen and sulfur on yield, yield components, some chemical composition and nutritional quality of canola plant grown in saline soil condition. Res J Pharm Biochem Sci 6:1055–1064
Anderson JW, Fitzgerald MA (2003) Sulphur distribution and redistribution. In: Abrol YP, Ahmad A (eds) Sulphur in plants. Kluwer, Dordrecht, pp 113–134
Anjum NA, Gill SS, Umar S, Ahmad I, Duarte AC, Pereira E (2012) Improving growth and productivity of Oleiferous Brassicas under changing environment: significance of nitrogen and sulphur nutrition, and underlying mechanisms. Sci World J. https://doi.org/10.1100/2012/657808
AOAC (Association of Official Analytical Chemists). (1984). OfficialMethods of Analysis(14th ed). VA, USA: Arlington
Astolfi S, Zuchi S, Neumann G, Cesco S, di Toppi L, Pinton R (2012) Response of barley plants to Fe deficiency and Cd contamination as affected by S starvation. J Exp Bot 633:1241–1250. https://doi.org/10.1093/jxb/err344
Banerjee A, Roychoudhury A (2019) Role of selenium in plants against abiotic stresses: phenological and molecular aspects. In: Roychoudhury A, Tripathi D (eds) Molecular plant abiotic stress, Wiley, pp 123–133
Bäucker E, Köhler R, Matschullat J, Wienhaus O, Zimmermann F (2003) Sulphate and cation accumulation in mesophyll and endodermis vacuoles of spruce needles–effects on nutrient supply after SO2 fumigation. Trees 17:1–10. https://doi.org/10.1007/s00468-002-0199-x
Becana M, Wienkoop S, Matamoros MA (2018) Sulfur transport and metabolism in legume root nodules. Front Plant Sci 9:1434
Bocchini M, D’Amato R, Ciancaleoni S, Fontanella MC, Palmerini CA, Beone GM, Onofri A, Negri V, Marconi G, Albertini E, Businelli D (2018) Soil selenium (Se) biofortification changes the physiological, biochemical and epigenetic responses to water stress in Zea mays L. by inducing a higher drought tolerance. Front Plant Sci 9:389
Chandra N, Pandey N (2016) Role of sulfur nutrition in plant and seed metabolism of Glycine max L. J Plant Nutr 39:1103–1111
Chauhan R, Awasthi S, Srivastava S, Dwivedi S, Pilon-Smits EA, Dhankher OP, Tripathi RD (2019) Understanding selenium metabolism in plants and its role as a beneficial element. Critic Rev Environ Sci Technol 49:1937–1958
Chen PS, Toribara TT, Warner H (1956) Microdetermination of phosphorus. Anal Chem 28:1756–1758
Chen J, Shafi M, Li S, Wang Y, Wu J, Ye Z, Peng D, Yan W, Liu D (2015) Copper induced oxidative stresses, antioxidant responses and phytoremediation potential of Moso bamboo (Phyllostachys pubescens). Sci Rep 5:13554
Chutipaijit S, Chaum S, Sompornpailin K (2011) High contents of proline and anthocyanin increase protective response to salinity in Oryza sativa L. spp. indica. Aus J Crop Sci 5: 1191
De Bona FD, Fedoseyenko D, von Wirén N, Monteiro FA (2011) Nitrogen utilization by sulfur-deficient barley plants depends on the nitrogen form. Environ Exp Bot 74:237–244
de Figueiredo MA, Boldrin PF, Hart JJ, de Andrade MJ, Guilherme LR, Glahn RP, Li L (2017) Zinc and selenium accumulation and their effect on iron bioavailability in common bean seeds. Plant Physiol Biochem 111:193–202
Dinh QT, Wang M, Tran TAT, Zhou F, Wang D, Zhai H, Peng Q, Xue M, Du Z, Bañuelos GS, Lin ZQ (2019) Bioavailability of selenium in soil-plant system and a regulatory approach. Critic Rev Environ Sci Technol 49:443–517
Djanaguiraman M, Prasad PVV, Seppanen M (2010) Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol Biochem 48:999–1007
Eich-Greatorex S, Krogstad T, Sogn TA (2010) Effect of phosphorus status of the soil on selenium availability. J Plant Nutr Soil Sci 173:337–344
Ernst L, Goodger JQ, Alvarez S, Marsh EL, Berla B, Lockhart E, Jung J, Li P, Bohnert HJ, Schachtman DP (2010) Sulphate as a xylem-borne chemical signal precedes the expression of ABA biosynthetic genes in maize roots. J Exp Bot 61:3395–3405
Fang X, Turner NC, Yan G, Li F, Siddique KHM (2010) Flower numbers, pod production, pollen viability, and pistil function are reduced and flower and pod abortion increased in chickpea (Cicer arietinum L.) under terminal drought. J Exp Bot 61:335–345. https://doi.org/10.1093/jxb/erp307
Fatma M, Asgher M, Masood A, Khan NA (2014) Excess sulfur supplementation improves photosynthesis and growth in mustard under salt stress through increased production of glutathione. Environ Exp Bot 107:55–63. https://doi.org/10.1080/15592324.2014.1003751
Fatma M, Masood A, Per TS, Khan NA (2016) Nitric oxide alleviates salt stress inhibited photosynthetic performance by interacting with sulfur assimilation in mustard. Front Plant Sci 7:521
Garg BK, Burman U, Kathju S (2006) Influence of thiourea on photosynthesis, nitrogen metabolism and yield of clusterbean (Cyamopsis tetragonoloba (L.) Taub.) under rainfed conditions of Indian arid zone. Plant Growth Regul 48:237–245
Gaur PM, Jukanti AK, Samineni S, Chaturvedi SK, Basu PS, Babbar A, Jeyalakshmi V, Nayyar H, Devasirvatham V, Mallikarjuna N (2012) Climate change and heat stress tolerance in chickpea. In: Tuteja N, Gill SS (eds) Climate change and plant abiotic stress tolerance. Wiley Blackwell, Weinheim, Germany, pp 839–855
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Giriappa S (1988) Water use efficiency in agriculture. In: Oxford and IBH Publishing Co., New Delhi, pp 6
Golob A, Kavčič J, Stibilj V, Gaberščik A, Vogel-Mikuš K, Germ M (2017) The effect of selenium and UV radiation on leaf traits and biomass production in Triticum aestivum L. Ecotoxicol Environ Saf 136:142–149
Guerrero B, Llugany M, Palacios O, Valiente M (2014) Dual effects of different selenium species on wheat. Plant Physiol Biochem 83:300–307
Habibi G (2013) Effect of drought stress and selenium spraying on photosynthesis and antioxidant activity of spring barley/Ucinek susnega stresa in skropljenja s selenom na fotosintezo in antioksidativno aktivnost jarega jecmena. Acta Agric Slov 101:31
Hajiboland R, Sadeghzadeh N, Ebrahimi N, Sadeghzadeh B, Mohammadi SA (2015) Influence of selenium in drought-stressed wheat plants under greenhouse and field conditions. Acta Agri Slov 105:175–191
Hajiboland R, Rahmat S, Zeinalzadeh N, Farsad-Akhtar N, Hosseinpour-Feizi MA (2019) Senescence is delayed by selenium in oilseed rape plants. J Trace Elem Med Bio 55:96–106
Hartikainen H (2005) Biogeochemistry of selenium and its impact on food chain quality and human health. J Trace Elem Med Bio 18:309–318
Havlin JL, Tisdale SL, Nelson WL, Beaton JD (2014) In: Soil fertility and fertilizers, 8th ed, New Jersey, USA: Prentice Hall
Honsel A, Kojima M, Haas R, Frank W, Sakakibara H, Herschbach C, Rennenberg H (2012) Sulphur limitation and early sulphur deficiency responses in poplar: significance of gene expression, metabolites, and plant hormones. J Exp Bot 63:1873–1893
Hussain RA, Ahmad R, Nawaz F, Ashraf MY, Warraich EA (2016) Foliar NK application mitigates drought effects in sunflower (Helianthus annuus L.). Acta Physiol Plantar 38: 83
Ilbas AI, Yilmaz S, Akbulut M, Bogdevich O (2012) Uptake and distribution of selenium, nitrogen and sulfur in three barley cultivars subjected to selenium applications. J Plant Nutr 35:442–452
Jackson ML, Barak P (2005) Soil chemical analysis: advanced course. UW-Madison Libraries Parallel Press, Madison (WI)
Karl TR, Melillo JM, Peterson TC (2009) Global climate change impacts in the United States. Cambridge University Press, Cambridge
Karpiuk UV, Al Azzam KM, Abudayeh ZH, Kislichenko V, Naddaf A, Cholak I, Yemelianova O (2016) Qualitative and quantitative content determination of macro-minor elements in Bryonia alba L. roots using flame atomic absorption spectroscopy technique. Adv Pharm Bull 6:285.
Kaur G, Asha W, Abdin MZ, Sarwat M, Ahmad A (2013) Molecular Network of Nitrogen and Sulphur Signaling in Plants. In: Sarwat et al. (eds.) Stress signaling in plants: genomics and proteomics perspective, vol. 1, Springer, New York, pp 240
Kaur M, Sharma S, Singh D (2018) Influence of selenium on carbohydrate accumulation in developing wheat grains. Commun Soil Sci Plant Anal 49:1650–1659
Kornaś A, Filek M, Sieprawska A, Bednarska-Kozakiewicz E, Gawrońska K, Miszalski Z (2019) Foliar application of selenium for protection against the first stages of mycotoxin infection of crop plant leaves. J Sci Food Agric 99:482–485
Kudre TG, Benjakul S, Kishimura H (2013) Comparative study on chemical compositions and properties of protein isolates from mung bean, black bean and bambara groundnut. J Sci Food Agric 93:2429–2436
Kutman UB, Yildiz B, Cakmak I (2011) Effect of nitrogen on uptake, remobilization and partitioning of zinc and iron throughout the development of durum wheat. Plant Soil 342:149–164
Lee BR, Jin YL, Avice JC, Cliquet JB, Ourry A, Kim TH (2009) Increased proline loading to phloem and its effect on nitrogen uptake and assimilation in water stressed white clover (Trifolium repens). New Phytol 182:654–663. https://doi.org/10.1111/j.1469-8137.2009.02795.x
Lee BR, Zaman R, Avice JC, Ourry A, Kim TH (2015) Sulfur use efficiency is a significant determinant of drought stress tolerance in relation to photosynthetic activity in Brassica napus cultivars. Front Plant Sci 7:459
Lefsrud MG, Kopsell DA, Kopsell DE, Curran-Celentano J (2006) Irradiance levels affect growth parameters and carotenoid pigments in kale and spinach grown in a controlled environment. Physiol Plantar 127:624–631
Leng G (2017) Evidence for a weakening strength of temperature-corn yield relation in the United States during 1980–2010. Sci Total Environ 605:551–558
Leng G, Hall J (2019) Crop yield sensitivity of global major agricultural countries to droughts and the projected changes in the future. Sci Total Environ 654:811–821
Lu Y (2018) Assembly and transfer of iron–sulfur clusters in the plastid. Front Plant Sci 9:336
Majeed S, Nawaz F, Naeem M, Ashraf MY, Ejaz S, Ahmad KS, Tauseef S, Farid G, Khalid I, Mehmood K (2020) Nitric oxide regulates water status and associated enzymatic pathways to inhibit nutrients imbalance in maize (Zea mays L.) under drought stress. Plant Physiol Biochem 155:147–160
Malik JA, Goel S, Kaur N, Sharma S, Singh I, Nayyar H (2012) Selenium antagonises the toxic effects of arsenic on mungbean (Phaseolus aureus Roxb.) plants by restricting its uptake and enhancing the antioxidative and detoxification mechanisms. Environ Exp Bot 77:242–248
Muneer S, Lee BR, Kim KY, Park SH, Zhang Q, Kim TH (2014) Involvement of sulphur nutrition in modulating iron deficiency responses in photosynthetic organelles of oilseed rape (Brassica napus L.). Photosynth Res 119:319–329. https://doi.org/10.1007/s11120-013-9953-8
Nadeem M, Li J, Yahya M, Sher A, Ma C, Wang X, Qui L (2019) Research progress and perspective on drought stress in legumes. Rev Int J Mol Sci 20:1–32. https://doi.org/10.3390/ijms20102541
Nair RM, Pandey AK, War AR, Hanumantharao B, Shwe T, Alam AKMM, Pratap A, Malik SR, Karimi R, Mbeyagala EK, Douglas CA (2019) Biotic and Abiotic Constraints in Mungbean Production—Progress in Genetic Improvement. Front Plant Sci 10.
Nawaz F, Ashraf MY, Ahmad R, Waraich EA (2013) Selenium (Se) seed priming induced growth and biochemical changes in wheat under water deficit conditions. Biol Trace Elem Res 151:284–293
Nawaz F, Ahmad R, Ashraf MY, Waraich EA, Khan SZ (2015a) Effect of selenium foliar spray on physiological and biochemical processes and chemical constituents of wheat under drought stress. Ecotox Environ Saf 113:191–200
Nawaz F, Ashraf MY, Ahmad R, Waraich EA, Shabbir RN, Bukhari MA (2015b) Supplemental selenium improves wheat grain yield and quality through alterations in biochemical processes under normal and water deficit conditions. Food Chem 175:350–357
Nazar R, Iqbal N, Masood A, Syeed S, Khan NA (2011) Understanding the significance of sulphur in improving salinity tolerance in plants. Environ Exp Bot 70:80–87. https://doi.org/10.1016/j.envexpbot.2010.09.011
Noctor G, Mhamdi A, Chaouch S, Han YI, Neukermans J, Marquez-Garcia BELEN, Queval G, Foyer CH (2012) Glutathione in plants: an integrated overview. Plant Cell Environ 35:454–484
Proietti P, Nasinia L, Del Buonoa D, D’Amatoa R, Tedeschinib E, Businellia D (2013) Selenium protects olive (Olea europaea L.) from drought stress. Sci Hortic 164:165–171
Raina SK, Govindasamy V, Kumar M, Singh AK, Rane J, Minhas PS (2016) Genetic variation in physiological responses of mungbeans (Vigna radiata (L.) Wilczek) to drought. Acta Physiol Plantar 38:263. doi: https://doi.org/10.1007/s11738-016-2280-x
Ramos SJ, Rutzke MA, Hayes RJ, Faquin V, Guilherme LRG, Li L (2011) Selenium accumulation in lettuce germplasm. Planta 233:649–660
Rayman MP (2008) Food-chain selenium and human health: emphasis on intake. Br J Nutr 100:254–268
Reeves MA, Hoffmann PR (2009) The human selenoproteome: recent insights into functions and regulation. Cell Mol Life Sci 66:2457–2478
Reich M, Shahbaz M, Prajapati DH, Parmar S, Hawkesford MJ, De Kok LJ (2016) Interactions of sulfate with other nutrients as revealed by H2S fumigation of Chinese cabbage. Front Plant Sci 7:541
Reis AFB, Almeida REM, Lago BC, Trivelin PC, Linquist BA, Favarin JL (2018) Aerobic rice system improves water productivity, nitrogen recovery and crop performance in Brazilian weathered lowland soil. Field Crops Res 218:59–68
Rossi L, Fedenia LN, Sharifan H, Ma X, Lombardini L (2018) Effects of foliar application of zinc sulfate and zinc nanoparticles in coffee (Coffea arabica L.) plants. Plant Physiol Biochem 135:160–166
Rubiales D, Mikić A (2015) Introduction: legumes in sustainable agriculture. Critic Rev Plant Sci 34:2–3
Sabatino L, Ntatsi G, Iapichino G, D’Anna F, De Pasquale C (2019) Effect of selenium enrichment and type of application on yield, functional quality and mineral composition of curly endive grown in a hydroponic System. Agronomy 9:207
Sachan HK, Krishna D, Chaudhary NK (2019) Sulphur Fertilization Effects on Yield and Nutrient Uptake of Mung bean [Vigna radiata (L.) Wilczek]. Ind J Agric Res doi: https://doi.org/10.18805/IJARe.A-5399
Salvagiotti F, Miralles DJ (2008) Radiation interception, biomass production and grain yield as affected by the interaction of nitrogen and sulfur fertilization in wheat. Eur J Agron 28:282–290
Schonfeld MA, Johnson RC, Carver BF, Mornhinweg DW (1988) Water relations in winter wheat as drought resistance indicators. Crop Sci 28:526–531
Schultz HR (2003) Differences in hydraulic architecture account for near-isohydric and anisohydric behaviour of two field-grown Vitis vinifera L. cultivars during drought. Plant Cell Environ 26:1393–1405
Sehgal A, Sita K, Siddique KH, Kumar R, Bhogireddy S, Varshney RK, HanumanthaRao B, Nair RM, Prasad PV, Nayyar H (2018) Drought or/and heat-stress effects on seed filling in food crops: impacts on functional biochemistry, seed yields, and nutritional quality. Front Plant Sci 9:1705
Shahbaz M, Stuiver CEE, Posthumus FS, Parmar S, Hawkesford MJ, De Kok LJ (2014) Copper toxicity in Chinese cabbage is not influenced by plant sulphur status, but affects sulphur metabolism-related gene expression and the suggested regulatory metabolites. Plant Biol 16:68–78
Shahid MA, Balal RM, Khan N, Zotarelli L, Liu GD, Sarkhosh A, Fernández-Zapata JC, Nicolás JJM, Garcia-Sanchez F (2019) Selenium impedes cadmium and arsenic toxicity in potato by modulating carbohydrate and nitrogen metabolism. Ecotoxicol Environ Saf 180:588–599
Shehzad MA, Nawaz F, Ahmad F, Ahmad N, Masood S (2020) Protective effect of potassium and chitosan supply on growth, physiological processes and antioxidative machinery in sunflower (Helianthus annuus L.) under drought stress. Ecotoxicol Environ Saf 187: 109841
Shekari L, Aroiee H, Mirshekari A, Nemati H (2019) Protective role of selenium on cucumber (Cucumis sativus L.) exposed to cadmium and lead stress during reproductive stage role of selenium on heavy metals stress. J Plant Nutr 42:529–542
Shekhawat K, Shivay YS (2012) Residual effects of nitrogen sources, sulfur and boron levels on mungbean (Vigna radiata) in a sunflower (Helianthus annuus)–mungbean system. Arch Agron Soil Sci 58:765–776
Singh DP, Singh BB (2011) Breeding for tolerance to abiotic stresses in mung bean. J Food Legumes 24:83–90
Sors TG, Ellis DR, Na GN, Lahner B, Lee S, Leustek T, Pickering IJ, Salt DE (2005) Analysis of sulfur and selenium assimilation in Astragalus plants with varying capacities to accumulate selenium. Plant J 42:785–797
Speiser A, Silbermann M, Dong Y, Haberland S, Uslu VV, Wang S, Bangash SA, Reichelt M, Meyer AJ, Wirtz M, Hell R (2018) Sulfur partitioning between glutathione and protein synthesis determines plant growth. Plant Physiol 177:927–937
Stuiver CEE, Posthumus FS, Parmar S, Shahbaz M, Hawkesford MJ, Kok LJD (2014) Zinc exposure has differential effects on uptake and metabolism of sulfur and nitrogen in Chinese cabbage. J Plant Nutr Soil Sci 177:748–757
Taalab AS, Hellal FA, Abou-Seeda MA (2008) Influence of phosphatic fertilizers enriched with sulfur on phosphorus availability and corn yield in calcareous soil in arid region. Open J Appl Sci 1:105–115
Tabak M, Lepiarczyk A, Filipek-Mazur B, Bachara P (2019) Ammonium nitrate enriched with sulfur influences wheat yield and soil properties. Plant Soil Environ 65:211–217
Troy TJ, Kipgen C, Pal I (2015) The impacts of climate extremes and irrigation on US crop yields. Environ Res Lett 10:054013. https://doi.org/10.1088/1748-9326/10/5/054013
Turkan I, Bor M, Ozdemir F, Koca H (2005) Differential responses of lipid peroxidation and antioxidants in the leaves of drought-tolerant P. acutifolius Gray and drought sensitive P. vulgaris L. subjected to polyethylene glycol mediated water stress. Plant Sci 168:223–231
Urbanek H, Kuzniak-Gebarowska E, Herka K (1991) Elicitation of defence responses in bean leaves by Botrytis cinereapolygalacturonase. Acta Physiol Plantar 13:43–50
Urton R, Hangs RD, Schoenau JJ, Grant CA (2018) The response of a high-yielding canola hybrid to sulfur fertilization in three contrasting Saskatchewan soils. J Plant Nutr 41:1957–1969
Usmani MM, Nawaz F, Majeed S, Shehzad MA, Ahmad KS, Akhtar G, Aqib M, Shabbir RN (2020) Sulfate-mediated Drought tolerance in Maize involves Regulation at physiological and Biochemical Levels. Sci Rep 10:1–13
Van Rossum MWPC, Alberda M, van der Plas LHW (1997) Role of oxidative damage in tulip bulb scale micropropagation. Plant Sci 130:207–216
Vazin F (2012) Effect of zinc sulfate on quantitative and qualitative characteristics of corn (Zea mays) in drought stress. Cercet Agron Moldov 45:15–24
White PJ, Bowen HC, Parmaguru P, Fritz M, Spracklen WP, Spiby RE, Meacham MC, Mead A, Harriman M, Trueman LJ, Smith BM (2004) Interactions between selenium and sulphur nutrition in Arabidopsis thaliana. J Exp Bot 55:1927–1937
Yao X, Chu J, Liang L, Geng W, Li J, Hou G (2012) Selenium improves recovery of wheat seedlings at rewatering after drought stress. Russ J Plant Physiol 59:701–707
Zhao Q, Wu Y, Gao L, Ma J, Li CY, Xiang CB (2014) Sulfur nutrient availability regulates root elongation by affecting root indole-3-acetic acid levels and the stem cell niche. J Integr Plant Bio 56:1151–1163
Acknowledgements
Financial support as a Georg Forster postdoctoral fellowship to Dr. Fahim Nawaz by Alexander von-Humboldt (AvH) Foundation, Germany is gratefully acknowledged.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aqib, M., Nawaz, F., Majeed, S. et al. Physiological insights into sulfate and selenium interaction to improve drought tolerance in mung bean. Physiol Mol Biol Plants 27, 1073–1087 (2021). https://doi.org/10.1007/s12298-021-00992-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-021-00992-6