Abstract
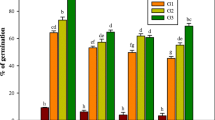
An efficient plant regeneration protocol was developed from leaf explants of Aloe barbadensis Mill on Murashige and Skoog’s (MS) medium supplemented with 2.0 mg/l 6-benzyladenine (BA) or Kinetin (Kn), 0.25–0.5 mg/l NAA (1-napthalene acetic acid) and 3 % (w/v) sucrose within 4 weeks of culture. The maximum number of shoot buds were obtained on MS medium supplemented with 2.0 mg/l BA, 0.5 mg/l NAA, 40 mg/l Ads (adenine sulphate) within 4–6 weeks of subculture. Inclusion of 0.25–0.50 mg/l gibberellic acid into the medium, the shoot buds became elongated. Repeated subculture on regeneration medium induces higher rate of shoot regeneration. The root induction from excised microshoots was achieved on half-strength MS medium supplemented with 0.25–1.0 mg/l NAA or indole-3-butyric acid (IBA) and 2 % (w/v) sucrose. Maximum percentage of rooting was achieved on medium having 0.5 mg/l NAA with 3 % (w/v) sucrose. About 80 % of in vitro raised plantlets were hardened in the greenhouse and successfully established in the soil. Both Random Amplified Polymorphic DNA (RAPD) and Inter Simple Sequence Repeat (ISSR) markers were used to detect the variability among the regenerated plants developed in vitro. The results showed that there was no polymorphism among the regenerated plantlets. This study will help for propagation of quality planting material of Aloe barbadensis for commercialization.



Similar content being viewed by others
References
Aggarwal D, Bama KS (2004) Tissue culture propagation of elite plant of Aloe vera L. J Plant Biochem Biotechnol 13:77–79
Baksha R, Jahan MAA, Khatun R, Munshi JL (2005) Micropropagation of Aloe barbadensis Mill. through in vitro culture of shoot tip explants. Plant Tissue Cult Biotechnol 15(2):121–126
Bantawa P, Saha-Roy O, Kumar S, Ghosh R, Mondal KT (2011) In vitro regeneration of an endangered medicinal plant Picrorhiz scrophulariiflora. Biol Plant 55:169–172
Budhiani E (2001) Micropropagation of Aloe vera through shoot multiplication. UG Thesis, Indonesia
Chaudhary AK, Ray AK, Jha S, Mishra IN (2011) Callus formation, shoot initiation and in vitro culture of Aloe vera. Biotechnol Bioinforma Bioeng 1(4):551–553
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Gui YI, Xu TY, Gu SR, Liu SQ, Zhang Z, Sun GD, Zhing Q (1990) Studies on stem tissue culture and organogenesis of Aloe vera. Acta Bot Sin 32:606–610
Harirah AA, Khalid N (2006) Direct regeneration and RAPD assessment of male inflorescence derived plants of Musa acuminata Cv. Berangan. Asia Pac J Mol Biol Biotechnol 14:11–17
Harter HL (1960) Critical values for Duncan’s multiple range test. Biogeosciences 16:671–685
Hashemabadi D, Kaviani B (2008) Rapid micropropagation of Aloe vera L. via shoot multiplication. Afr J Biotechnol 7(12):1899–1902
Hashemabadi D, Kaviani B (2010) In vitro proliferation of an important medicinal plant Aloe- A method for rapid production. Aust J Crop Sci 4(4):216–222
Jayakrishna C, Karthik C, Barathi S, Kamalanathan D, Indra Arulselvi P (2011) In vitro propagation of Aloe barbadensis Miller, a miracle herb. Res Plant Biol 1(5):22–26
Kathi JK, Victoria C (1999) The Longwood Herbal Task Force and the Centre for Holistic Pediatric Education and Research. Aloe Vera 2:29–34
Liao Z, Chen M, Tan F, Sun X, Tang K (2004) Micropropagation of endangered Chinese aloe. Plant Cell Tissue Organ Cult 78:83–86
Martins M, Sarmento D, Oliveira MM (2004) Genetic stability of micropropagated almond plantlets, as assessed by RAPD and ISSR markers. Plant Cell Rep 23:492–496
Meyer HJ, Staden J (1991) Rapid in vitro propagation of Aloe barhadensis Mill. Plant Cell Tissue Organ Cult 76(1):83–86
Mohanty S, Panda MK, Sahoo S, Nayak S (2011) Micropropagation of Zingiber rubens and assessment of genetic stability through RAPD and ISSR markers. Biol Plant 55:16–20
Murashige T, Skoog T (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol 15:473–497
Natali L, Sanchez IC, Cavallini (1990) In vitro culture of Aloe barbadensis Mill. micropropagation from vegetative meristems. Plant Cell Tissue Organ Cult 20:71–74
Premvaranon P, Vearasilp S, Thanapornpoong S, Karladee D, Gorinstein S (2011) In vitro studies to produce double haploid in Indica hybrid rice. Biologia 66:1074–1081
Rahman M, Rajora O (2001) Microsatellite DNA somaclonal variations in micropropagated trembling aspen (Populus tremuloides). Plant Cell Rep 20:531–536
Rout GR (2002) Direct plant regeneration from leaf explants of plumbago species and its genetic fidelity through RAPD markers. Ann Appl Biol 140:305–313
Rout GR, Das P, Goel S, Raina SN (1998) Determination of genetic stability of micropropagated plants of ginger using random amplified polymorphic DNA (RAPD) markers. Bot Bull Acad Sin 39:23–27
Rout GR, Mahato A, Senapati SK (2008) In vitro clonal propagation of Nyctanthes arbortristis. Biol Plant 52:521–524
Senapati S, Aparajita S, Rout GR (2013) Micropropagation and assessment of genetic stability in Celastrus paniculatus: an endangered medicinal plant. Biologia 68(4):627–632
Swarna J, Ravindhran R (2012) In vitro propagation and assessment of genetic integrity of Talinum triangulare (Jacq.) Wild: a valuable medicinal herb. Acta Physiol Plant 10:1007–1017
Vendrame WA, Kochert G, Wetzstein HY (1999) AFLP analysis of variation in pecan somatic embryos. Plant Cell Rep 18:853–857
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sahoo, S., Rout, G.R. Plant regeneration from leaf explants of Aloe barbadensis Mill. and genetic fidelity assessment through DNA markers. Physiol Mol Biol Plants 20, 235–240 (2014). https://doi.org/10.1007/s12298-014-0226-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-014-0226-6