Abstract
Chronic kidney disease (CKD) is a complex health condition characterized by the gradual loss of renal function, often leading to end-stage renal disease (ESRD). It results from a combination of medical, environmental, and genetic factors. Predicting the rate of renal function decline and effectively managing the progression to ESRD is challenging in clinical practice. CKD assessment involves various indicators, including estimated glomerular filtration rate (eGFR), albuminuria levels, serum creatinine, and others. This study aimed to explore the predictive potential of specific blood cell indexes in forecasting further renal function decline and the transition from CKD stage 3–4 to ESRD. We assessed the following blood cell indexes in 377 CKD stage 3–4 patients: absolute neutrophil count (ANC), neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), derived NLR (dNLR), mean platelet volume (MPV), aggregate index of systemic inflammation (AISI), and systemic inflammation index (SII). ANC, MPV, NLR, PLR, dNLR, and SII were found to independently predict a rapid decline in eGFR. Notably, NLR and dNLR demonstrated the highest sensitivity and specificity with cut-off values of 3.36 and 2.45, respectively (NLR: 88.6 and 81.7%; dNLR: 85.2 and 75.8%). The corresponding area under the ROC curve values were 0.877 (95% CI 0.837–0.918, p < 0.001) for NLR and 0.849 (95% CI 0.805–0.892, p < 0.001) for dNLR. However, none of the blood cell indexes independently predicted the transition to ESRD. The NLR and the dNLR exhibited the highest predictive capacity towards a rapid decline in renal function in CKD. No blood cell index, however, independently predicted the transition into ERSD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of chronic kidney disease (CKD) varies across different regions and is influenced by a range of factors, including population demographics, access to healthcare, and lifestyle choices [1]. Recognized as a global health concern by organizations such as the World Health Organization (WHO) and the International Society of Nephrology, CKD has shown a consistent increase in prevalence in many parts of the world. Globally, CKD affects more than 10% of the population to varying degrees, but the specific prevalence rates can differ significantly from one country or region to another [2]. Notably, low- and middle-income countries may experience higher prevalence rates due to challenges like limited access to healthcare services, the presence of infectious diseases, and environmental factors [3]. In the United States alone, CKD is a significant public health issue. According to estimates provided by the National Kidney Foundation and the Centers for Disease Control and Prevention (CDC), approximately 37 million adults in the U.S. were living with CKD, representing around 15% of the adult population. Ageing, diabetes, obesity, and hypertension are considered the main causes of CKD, and place a considerable financial burden in most advanced healthcare systems [2, 3]. Moreover, the number of deaths due to poor access to renal replacement therapy in developing countries remains unacceptably high [4].
Nephron loss triggers hypertrophy and hyperfiltration in remaining nephrons, and even with successful management of diabetes and hypertension, CKD often advances to end-stage renal disease (ESRD) affecting an estimated 4.902–7.083 million individuals worldwide. CKD has become a significant global health concern, with a prevalence of approximately 13.4%, impacting morbidity and mortality on a global scale. Its growth is primarily driven by factors such as diabetes, hypertension, obesity, and an aging population, though specific causes may vary regionally, including infections and environmental factors [4, 5]. However, significant challenges exist in clinical practice regarding the capacity to predict the temporal progression of CKD, preventing the institution of suitable preventive and management strategies. CKD is characterized by chronic low-grade inflammation, endothelial dysfunction, and platelet activation [6, 7]. The inflammatory state in CKD is associated with the release of cytokines and the production and activation of adhesion molecules that lead to T-cell adhesion and migration into the interstitium, with consequent stimulation of pro-fibrotic factors. Inflammation in CKD increases the risk of cardiovascular events, all-cause mortality, and its progression by contributing to the development of vascular calcifications and endothelial dysfunction [6]. Although several inflammatory markers have been investigated in CKD, such as CRP, erythrocyte sedimentation rate, interleukin (IL)-6, IL-8, IL-12, tumor necrosis factor (TNF)-alpha, and IL-33, their ability to predict disease progression is unclear. In recent years, routinely measured blood cell indexes in several diseases [8,9,10,11,12] as the neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), monocyte/lymphocyte ratio (MLR), derived NLR (dNLR), systemic inflammation index (SII), and aggregate index of systemic inflammation (AISI) have been investigated as markers of inflammation in renal disease [13] and acute kidney injury after major abdominal surgery [14, 15]. However, their clinical significance has primarily been investigated in cancer and cardiovascular disease [16,17,18,19,20]. In this study we investigated the ability of these blood cell indexes to predict (a) the further decline in renal function in CKD and (b) the transition of CKD stage 3–4 to ESRD.
Methods
We conducted a retrospective study of CKD stage 3–4 patients (defined as an e-GFR between 30–60 and 15–30 ml/min/1.73 m2, respectively); including patients with comorbidities such as type 2 diabetes, hypertension, coronary artery disease, and the elderly attending the nephrology outpatient clinic at the Hue Central Hospital in Viet Nam from October 2015 to October 2018. Sample size: the required sample sizes for estimating specificity concerning the pre-determined value of specificity ascertained by previously published data [21].
P = 0.69, is a pre-determined value of specificity from published data of Ismail Kocyigit et al. [22]; α = 0.05; marginal error d = 5%. Sample size n = 329.
Exclusion criteria were follow-up time <1 year, eGFR > 60 ml/min/1.73 m2, acute inflammation (defined by a WBC count>15,000/mm3 and/or a C-reactive protein (CRP) concentration > 50 mg/dL), hematological diseases, other chronic inflammatory processes (such as rheumatoid arthritis, ankylosing spondylitis, and gout), and treatment with immunosuppressive therapies. We also excluded CKD patients exhibiting changes in eGFR to values compatible with stages less than 3 or 4 during follow-up, leaving 377 patients with CKD stages 3–4 suitable for analysis.
Patients underwent clinical and biochemical profile assessments to evaluate CKD progression, including CRP, urea, and creatinine. NLR (neutrophil to lymphocyte ratio), PLR (platelet to lymphocyte ratio), MLR (monocyte to lymphocyte ratio), dNLR (derived NLR), RDW (Red Cell Distribution With), AISI (aggregate index of systemic inflammation, calculated by multiplying the counts of neutrophils, monocytes, and platelets and then dividing the product by the lymphocyte count), and SII (systemic inflammation index, calculated by (neutrophil counts × platelet counts)/lymphocyte counts) were assessed at baseline and then serially during outpatient visits. Smoking status, cause of CKD, type of antihypertensive drugs, and laboratory tests were also recorded during the first visit. The time point of the last outpatient visit within the 3 years was defined as the last assessment. ESRD is defined as an irreversible decline in kidney function, which requires dialysis or transplantation to improve the glomerular filtration rate (GFR) [23]. For patients who transitioned to ESRD before the last outpatient visit, the last assessment was defined as the time point of transition. Therefore, the time to transition to ESRD (months) was the time interval between the first visit and the time point of transition to ESRD.
Estimated glomerular filtration rate (eGFR) was calculated using the MDRD equation [24]. According to KDIGO (International Kidney Disease: Improving Global Outcomes) 2012 guideline classification CKD stage 3 and 4 were defined as an eGFR between 30–60 and 15–30 ml/min/1.73 m2, respectively [23]. eGFR decline, the primary endpoint of the study, was defined as rapid or slow if the eGFR reduction was either >= 5 mL/min/year or < 5 mL/min/year, respectively, according to the following formula: annual progression rate (mL/min/year) = baseline eGFR-last eGFR (ml/min/1.73 m2) ×12 months/number of months [25].
Transition to ESRD, or initiation of renal replacement therapy, was the secondary endpoint to categorize the patients. Patients with an eGFR less than 15 ml/min/1.73 m2 are considered to be in an advanced state of renal failure and are often in need of dialysis or kidney transplantation, whereas Patients with an eGFR between 15 and 60 ml/min/1.73 m2 may have a variety of renal conditions but are not yet considered to be in transition.
Statistical Analysis
We expressed categorical data as percentage and number. Non-normally distributed variables were presented as median (range) and normally distributed variables as mean ± SD, as appropriate. Statistical significance was concluded when p-value < 0.05. We used the Chi-square test to assess between-group comparisons for nominal variables and the Independent Samples T-test to compare means between groups. Spearman’s rank correlation was used to determine correlations between paired variables. Variables showing associations with p-values <0.05 were included in logistic regression to determine their ability to independently predict the decline in eGFR and the transition to ESRD. We measured the ROC curve to determine the sensitivity, specificity, AUC, and cutoff values of predictive factors.
Ethical Standards
Compliance with ethical standards and approved by Ethic Council in biomedical research of Hue University of Medicine and Pharmacy, Vietnam. Date of Approval: 6 January, 2018.
Results
A total of 377 patients were included in the study (Table 1). There were no statistically significant differences in age, gender, BMI, smoking status, baseline serum creatinine, eGFR, type of antihypertensive drugs, blood cells (M, RDW, and PDW), and CRP concentrations between CKD patients with slow vs. rapid progression. There were significant differences in the duration of follow-up, causes of CKD, white blood cells (W), ANC(N), platelets (P), mean platelet volume (MPV), NLR, PLR, MLR, dNLR, SII, and AISI.
We run a correlation analysis between pre-specified variables and CKD progression rate. Variable showing associations with p-values < 0.05 were included in logistic regression to determine the independent predictive ability for the decline in eGFR and the transition to ESRD.
In univariate logistic regression analysis, the duration of follow-up, baseline eGFR, baseline WBC, baseline N, baseline L, baseline P, baseline MPV, baseline NLR, baseline PLR, baseline dNLR, baseline MLR, baseline SII, and baseline AISI were significantly associated with a rapid decline in eGFR. However, in multivariate analysis, only baseline N, baseline MPV, baseline NLR, baseline PLR, baseline dNLR, and baseline SII remained independent predictors of the rapid decline in eGFR (Table 2).
The ability of N, MPV, NLR, PLR, dNLR, and SII to predict a rapid decline in eGFR was further assessed by ROC analysis (Fig. 1). The sensitivity and specificity of NLR and dNLR were higher than the other indexes, 88.6 and 81.7%; 85.2 and 75.8%, respectively, using a cutoff level of 3.36, and 2.45, respectively. AUC areas of NLR and dNLR were 0.877 (95% CI 0.837–0.918, p < 0.001) and 0.849 (95% CI 0.805–0.892, p < 0.001), respectively. The detailed data of other indexes are described in Table 3.
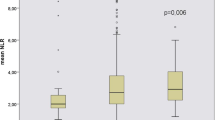
After a 3-year follow-up, 19.1% (72/377) CKD stage 3–4 transitioned to ESRD. There were no statistically significant differences in age, BMI, smoking status, duration of follow-up, type of antihypertensive drugs, causes of CKD, blood cells (W, N, M, P, MPV, RDW, and PDW), PLR and CPR concentrations between CKD patients with or without ESRD (Table 4). However, there were statistically significant differences in sex, baseline creatinine, eGFR, L, NLR, MLR, dNLR, SII, and AISI. Our results showed that patients transitioning to ESRD were also those with rapid eGFR deterioration (Fig. 2).
In univariate analysis, gender, baseline eGFR, kidney progression rate, baseline L, baseline P, baseline NLR, baseline NLR grade, baseline dNLR, baseline MLR, baseline SII, and baseline AISI were significantly associated with transition to ESRD. However, in multivariate analysis, only gender, baseline creatinine, kidney progression rate, and L had independent predictive roles in the transition to ESRD with p<0.001, p<0.001, p = 0.001 and p = 0.008, respectively) (Table 5).
Discussion
This study showed that N, MPV, NLR, PLR, dNLR, and SII significantly predicted a rapid decline in eGFR, with both the NLR and the dNLR exhibiting the highest predictive capacity based on AUC values. The decline in eGFR was faster in patients with high NLR (cutoff level > 3.36) and dNLR (cutoff level > 2.45) group, results similar to those of Kocyigit's et al. [22]. Neutrophils release chemotactic substances that promote neutrophil migration to the kidney, worsening glomerular injury. These changes contribute to the fast progression of CKD. NLR provide information regarding inflammation patients in CKD [26], predicts the severity and prognosis of several pathological conditions chronic [27, 28], NLR and dNLR predict survival in breast cancer [29], urological [30], colorectal, and gastrointestinal tumors [31], high pre-treatment NLR (≥4) predicted a poor prognosis in lung cancer [32]. NLR could be used to predict cardiovascular endpoints in CKD patients in moderate to severe conditions associated with endothelial dysfunction, predicts all-cause mortality in geriatric patients with CKD [33]. However, its predictive role in the progression of CKD has not been comprehensively investigated. Our study demonstrates that dNLR independently predicts rapid CKD progression. In CKD patients, inflammation is associated with pro-coagulant and platelet-activating factors contributing to endothelial dysfunction [34]. PLR was shown to be superior to NLR in terms of inflammation in ESRD patients [35] and could independently predict all-cause mortality in hemodialysis patients [36]. SII has been shown to be a useful indicator [37]. In our study, both predicted a rapid decline in eGFR with sensitivity and specificity were inferior to that of NLR and dNLR. Also, CRP has a significant role in the rapid decline in eGFR supporting the potential advantage of using NLR and dNLR as predictors of eGFR decline in CKD stage 3–4. The ability of blood count cells to predict the transition to ESRD also was assessed by logistic regression and ROC analysis. We did not identify any useful blood cell index to predict the transition to ESRD. Several studies are in agreement with our study, which has shown that the percentage of patients transitioning into ESRD was not different between high and low-baseline NLR groups [38] and revealed that NLR is associated with the risk of ESRD in Chinese patients with stage 4 CKD [39, 40]. More research is warranted to clarify the role of the NLR and other inflammatory indexes in the progression of CKD. The main limitation of our study is the retrospective design and the lack of all patient data on the inflammation marker CRP (Table 6).
Conclusion
In conclusion, our results suggest that the NLR and the dNLR have the highest predictive capacity of a rapid decline in eGFR and may represent useful markers for monitoring in CKD stage 3–4 in clinical practice because they are simple, inexpensive and easy to access.
References
Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl. 2022;12(1):7–11.
Collaborators GA. Global, regional, and national burden of diseases and injuries for adults 70 years and older: systematic analysis for the Global Burden of Disease 2019 Study. BMJ. 2022;376.
Basto-Abreu A, Barrientos-Gutierrez T, Wade AN, Oliveira de Melo D, Semeão de Souza AS, Nunes BP, Perianayagam A, Tian M, Yan LL, Ghosh A, Miranda JJ. Multimorbidity matters in low and middle-income countries. J Multimorb Comorb. 2022; 12:26335565221106074. https://doi.org/10.1177/26335565221106074. PMID: 35734547; PMCID: PMC9208045.
Lv J-C, Zhang L-X. Prevalence and disease burden of chronic kidney disease. Renal Fibrosis Mech Therapies. 2019:3–15.
Silverstein DM. Inflammation in chronic kidney disease: role in the progression of renal and cardiovascular disease. Pediatr Nephrol. 2009;24:1445–52.
Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, Zhao MH, Lv J, Garg AX, Knight J, Rodgers A, Gallagher M, Kotwal S, Cass A, Perkovic V. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385(9981):1975–82. https://doi.org/10.1016/S0140-6736(14)61601-9. (Epub 2015 Mar 13 PMID: 25777665).
Landray MJ, Wheeler DC, Lip GY, Newman DJ, Blann AD, McGlynn FJ, Ball S, Townend JN, Baigent C. Inflammation, endothelial dysfunction, and platelet activation in patients with chronic kidney disease: the chronic renal impairment in Birmingham (CRIB) study. Am J Kidney Dis. 2004;43(2):244–53.
Akchurin OM, Kaskel F. Update on inflammation in chronic kidney disease. Blood Purif. 2015;39(1–3):84–92.
Coradduzza D, Solinas T, Balzano F, Culeddu N, Rossi N, Cruciani S, Azara E, Maioli M, Zinellu A, De Miglio MR, Madonia M, Falchi M, Carru C. miRNAs as molecular biomarkers for prostate cancer. J Mol Diagn. 2022;24(11):1171–80. https://doi.org/10.1016/j.jmoldx.2022.05.005. (Epub 2022 Jul 11 PMID: 35835374).
Coradduzza D, Ghironi A, Azara E, Culeddu N, Cruciani S, Zinellu A, Maioli M, De Miglio MR, Medici S, Fozza C, Carru C. Role of polyamines as biomarkers in lymphoma patients: a pilot study. Diagnostics (Basel). 2022;12(9):2151. https://doi.org/10.3390/diagnostics12092151. PMID: 36140552; PMCID: PMC9497571.
Coradduzza D, Arru C, Culeddu N, Congiargiu A, Azara EG, Scanu AM, Zinellu A, Muroni MR, Rallo V, Medici S, Carru C, Angius A, De Miglio MR. Quantitative metabolomics to explore the role of plasma polyamines in colorectal cancer. Int J Mol Sci. 2022;24(1):101. https://doi.org/10.3390/ijms24010101.PMID:36613539;PMCID:PMC9820724.
Coradduzza D, Solinas T, Azara E, Culeddu N, Cruciani S, Zinellu A, Medici S, Maioli M, Madonia M, Carru C. Plasma polyamine biomarker panels: agmatine in support of prostate cancer diagnosis. Biomolecules. 2022;12(4):514. https://doi.org/10.3390/biom12040514.PMID:35454104;PMCID:PMC9024899.
Coradduzza D, Azara E, Medici S, Arru C, Solinas T, Madonia M, Zinellu A, Carru C. A preliminary study procedure for detection of polyamines in plasma samples as a potential diagnostic tool in prostate cancer. J Chromatogr B Analyt Technol Biomed Life Sci. 2021;1162: 122468. https://doi.org/10.1016/j.jchromb.2020.122468. (Epub 2020 Nov 30 PMID: 33370684).
Ergin G, Değer SM, Köprü B, Derici Ü, ARINSOY ST. High neutrophil-to- lymphocyte ratio predicts acute allograft rejection in kidney transplantation: a retrospective study. Turkish J Med Sci. 2019;49(2):525–30.
Gameiro J, Fonseca JA, Dias JM, Milho J, Rosa R, Jorge S, Lopes JA. Neutrophil, lymphocyte and platelet ratio as a predictor of postoperative acute kidney injury in major abdominal surgery. BMC Nephrol. 2018;19(1):320. https://doi.org/10.1186/s12882-018-1073-4.PMID:30419844;PMCID:PMC6231266.
Kolonko A, Dwulit T, Skrzypek M, Więcek A. Potential utility of neutrophil-to- lymphocyte, platelet-to-lymphocyte, and neutrophil, lymphocyte, and platelet ratios in differential diagnosis of kidney transplant acute rejection: a retrospective, propensity score matched analysis. Ann Transplant. 2022;27:e937239–41.
Catabay C, Obi Y, Streja E, Soohoo M, Park C, Rhee CM, Kovesdy CP, Hamano T, Kalantar-Zadeh K. Lymphocyte cell ratios and mortality among incident hemodialysis patients. Am J Nephrol. 2017;46(5):408–416. https://doi.org/10.1159/000484177. Epub 2017 Nov 7. PMID: 29130984; PMCID: PMC5777311.
Yilmaz G, Sevinc C, Ustundag S, Yavuz YC, Hacıbekiroglu T, Hatipoglu E, Baysal M. The relationship between mean platelet volume and neutrophil/lymphocyte ratio with inflammation and proteinuria in chronic kidney disease. Saudi J Kidney Dis Transpl. 2017;28(1):90–4.
Xiang F, Chen R, Cao X, Shen B, Liu Z, Tan X, Ding X, Zou J. Monocyte/lymphocyte ratio as a better predictor of cardiovascular and all-cause mortality in hemodialysis patients: A prospective cohort study. Hemodial Int. 2018;22(1):82–92. https://doi.org/10.1111/hdi.12549. (Epub 2017 Apr 12 PMID: 28403540).
Solak Y, Yilmaz MI, Sonmez A, Saglam M, Cakir E, Unal HU, Gok M, Caglar K, Oguz Y, Yenicesu M, Karaman M, Ay SA, Gaipov A, Turk S, Vural A, Carrero JJ. Neutrophil to lymphocyte ratio independently predicts cardiovascular events in patients with chronic kidney disease. Clin Exp Nephrol. 2013;17(4):532–40. https://doi.org/10.1007/s10157-012-0728-x. (Epub 2012 Nov 20 PMID: 23180042).
Tonyali S, Ceylan C, Yahsi S, Karakan MS. Does neutrophil to lymphocyte ratio demonstrate deterioration in renal function? Ren Fail. 2018;40(1):209–12.
Kocyigit I, Eroglu E, Unal A, Sipahioglu MH, Tokgoz B, Oymak O, Utas C. Role of neutrophil/lymphocyte ratio in prediction of disease progression in patients with stage-4 chronic kidney disease. J Nephrol. 2013;26(2):358–65. https://doi.org/10.5301/jn.5000152. (Epub 2012 May 8 PMID: 22573523).
Hajian-Tilaki K. Sample size estimation in diagnostic test studies of biomedical informatics. J Biomed Inform. 2014;48(2):193–204.
KDIGO. Clinical practice guideline for the evaluation and management of chronic kidney disease. Offic J Int Soc Nephrol. 2012; 3(1).
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–70.
Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–30.
Okyay GU, Inal S, Oneç K, Er RE, Paşaoğlu O, Paşaoğlu H, Derici U, Erten Y. Neutrophil to lymphocyte ratio in evaluation of inflammation in patients with chronic kidney disease. Ren Fail. 2013;35(1):29–36. https://doi.org/10.3109/0886022X.2012.734429. (Epub 2012 Nov 1 PMID: 23113674).
Yao C, Liu X, Tang Z. Prognostic role of neutrophil-lymphocyte ratio and platelet- lymphocyte ratio for hospital mortality in patients with AECOPD. Int J Chron Obstruct Pulmon Dis. 2017;12:2285–90.
Yang Z, Zhang Z, Lin F, Ren Y, Liu D, Zhong R, Liang Y. Comparisons of neutrophil-, monocyte-, eosinophil-, and basophil-lymphocyte ratios among various systemic autoimmune rheumatic diseases. APMIS. 2017;125(10):863–71. https://doi.org/10.1111/apm.12722. (Epub 2017 Aug 2 PMID: 28766758).
Krenn-Pilko S, Langsenlehner U, Stojakovic T, Pichler M, Gerger A, Kapp KS, Langsenlehner T. The elevated preoperative derived neutrophil-to-lymphocyte ratio predicts poor clinical outcome in breast cancer patients. Tumour Biol. 2016;37(1):361–8. https://doi.org/10.1007/s13277-015-3805-4. (Epub 2015 Jul 29 PMID: 26219894).
Van Soest RJ, Templeton AJ, Vera-Badillo FE, Mercier F, Sonpavde G, Amir E, Tombal B, Rosenthal M, Eisenberger MA, Tannock IF, de Wit R. Neutrophil-to-lymphocyte ratio as a prognostic biomarker for men with metastatic castration-resistant prostate cancer receiving first-line chemotherapy: data from two randomized phase III trials. Ann Oncol. 2015;26(4):743–9. https://doi.org/10.1093/annonc/mdu569. (Epub 2014 Dec 15 PMID: 25515657).
Absenger G, Szkandera J, Pichler M, Stotz M, Arminger F, Weissmueller M, Schaberl-Moser R, Samonigg H, Stojakovic T, Gerger A. A derived neutrophil to lymphocyte ratio predicts clinical outcome in stage II and III colon cancer patients. Br J Cancer. 2013;109(2):395–400. https://doi.org/10.1038/bjc.2013.346. Epub 2013 Jul 2. PMID: 23820252; PMCID: PMC3721404.
Yu Y, Qian L, Cui J. Value of neutrophil-to-lymphocyte ratio for predicting lung cancer prognosis: a meta-analysis of 7,219 patients. Molec Clinic Oncol. 2017;7(3):498–506.
Tatar E, Mirili C, Isikyakar T, Yaprak M, Guvercin G, Ozay E, Asci G. The association of neutrophil/lymphocyte ratio and platelet/lymphocyte ratio with clinical outcomes in geriatric patients with stage 3–5 chronic kidney disease. Acta Clin Belg. 2016;71(4):221–6. https://doi.org/10.1080/17843286.2016.1159797. (Epub 2016 May 20 PMID: 27309205).
Conway DS, Pearce LA, Chin BS, Hart RG, Lip GY. Plasma von Willebrand factor and soluble p-selectin as indices of endothelial damage and platelet activation in 1321 patients with nonvalvular atrial fibrillation: relationship to stroke risk factors. Circulation. 2002;106(15):1962–7.
Turkmen K, Erdur FM, Ozcicek F, Ozcicek A, Akbas EM, Ozbicer A, Demirtas L, Turk S, Tonbul HZ. Platelet-to-lymphocyte ratio better predicts inflammation than neutrophil-to-lymphocyte ratio in end-stage renal disease patients. Hemodial Int. 2013;17(3):391–6. https://doi.org/10.1111/hdi.12040. (Epub 2013 Mar 24 PMID: 23522328).
Yaprak M, Turan MN, Dayanan R, Akın S, Değirmen E, Yıldırım M, Turgut F. Platelet-to-lymphocyte ratio predicts mortality better than neutrophil-to-lymphocyte ratio in hemodialysis patients. Int Urol Nephrol. 2016;48(8):1343–8. https://doi.org/10.1007/s11255-016-1301-4. (Epub 2016 Apr 27 PMID: 27118565).
Feng JF, Chen S, Yang X. Systemic immune-inflammation index (SII) is a useful prognostic indicator for patients with squamous cell carcinoma of the esophagus. Medicine. 2017;96(4): e5886.
Altunoren O, Akkus G, Sezal DT, Ciftcioglu M, Guzel FB, Isiktas S, Torun GI, Uyan M, Sokmen MF, Sevim HA, Sarısık FN, Senel ME, Erken E, Gungor O. Does neutrophyl to lymphocyte ratio really predict chronic kidney disease progression? Int Urol Nephrol. 2019;51(1):129–37. https://doi.org/10.1007/s11255-018-1994-7. (Epub 2018 Oct 1 PMID: 30276600).
Yuan Q, Wang J, Peng Z, Zhou Q, Xiao X, Xie Y, Wang W, Huang L, Tang W, Sun D, Zhang L, Wang F, Zhao MH, Tao L, He K, Xu H; C-STRIDE study group. Neutrophil-to-lymphocyte ratio and incident end-stage renal disease in Chinese patients with chronic kidney disease: results from the Chinese Cohort Study of Chronic Kidney Disease (C-STRIDE). J Transl Med. 2019;17(1):86. https://doi.org/10.1186/s12967-019-1808-4. PMID: 30876475; PMCID: PMC6420746.
Acknowledgements
Duong Thi Ngoc Lan is supported by AISPO, a Non-Governmental Organization (NGO—nonprofit entity), Scientific Institute San Raffaele Hospital of Milan (Italy).
Funding
Open access funding provided by Università degli Studi di Sassari within the CRUI-CARE Agreement. This research received no external funding.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare no conflict of interest.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of Ethic council in biomedical research, Hue University of Medicine and Pharmacy, protocol code 1435/QD-DHYD on Januart 4th, 2018. Written informed consent has been obtained from the patients to publish this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lan, D.T.N., Coradduzza, D., Van An, L. et al. Role of Blood Cell Indexes in Progresses to ESRD. Ind J Clin Biochem (2024). https://doi.org/10.1007/s12291-024-01184-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12291-024-01184-1