Abstract
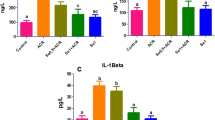
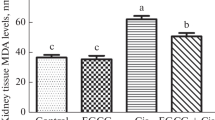
Exposure to various organic compounds including drugs and environmental toxins causes cellular damage through generation of free radicals. Carnosine a dipeptide was used in this study to evaluate its effect against CCl4-induced nephrotoxicity. Sixty male albino rats were involved in this study and were equally divided into four groups. CCl4 (3 ml/kg body weight; biweekly for 4 weeks) was given to group II and III. Carnosine (10 mg/kg body weight; once daily for 4 weeks) was given to group III and VI. Transforming growth factor-β1 (TGF-β1) level by immunoassay and Smad3 mRNA level by real-time PCR were estimated in addition to cytochrome P450 2E1 (CYP2E1) activity, renal functions, redox status assessment and histopathological examination of the kidney. Carnosine significantly improved kidney function, renal redox status, decreased renal CYP2E1 activity, TGF-β1 level and Smad3 gene expression when compared to CCL4-intoxicated group. The protective effect of carnosine was confirmed by histopathological study. In conclusion: carnosine has the ability to protect against CCl4-induced nephrotoxicity possibly by alleviating oxidative stress, normalizing kidney histopathological architecture in addition to the disruption of the inflammatory and fibrotic response induced by CCl4.




Similar content being viewed by others
References
Koc Y, Sokmen M, Unsal A, Cigerli S, Ozagari A, Basturk T, et al. Effects of human umbilical cord stem cells and granulocyte colony-stimulating factor (G-CSF) on carbon tetrachloride-induced nephrotoxicity. Nephrourol Mon. 2012;4(3):545–50. doi:10.5812/numonthly.2979.
Khan RA, Khan MR, Sahreen S. Evaluation of Launaea procumbens use in renal disorders: a rat model. J Ethnopharmacol. 2010;128(2):452–61. doi:10.1016/j.jep.2010.01.026.
Ma JQ, Ding J, Xiao ZH, Liu CM. Ursolic acid ameliorates carbon tetrachloride-induced oxidative DNA damage and inflammation in mouse kidney by inhibiting the STAT3 and NF-κB activities. Int Immunopharmacol. 2014;21(2):389–95. doi:10.1016/j.intimp.2014.05.022.
García-Sánchez O, López-Hernández FJ, López-Novoa JM. An integrative view on the role of TGF-beta in the progressive tubular deletion associated with chronic kidney disease. Kidney Int. 2010;77(11):950–5. doi:10.1038/ki.2010.88.
Noori S, Mahboob T. Antioxidant effect of carnosine pretreatment on cisplatin-induced renal oxidative stress in rats. Indian J Clin Biochem. 2010;25(1):86–91. doi:10.1007/s12291-010-0018-x.
Kiliś-Pstrusińska K. Carnosine, carnosinase and kidney diseases. Postepy Hig Med Dosw. 2012;66:215–21.
Khan RA, Khan MR, Sahreen S, Bokhari J. Prevention of CCl4-induced nephrotoxicity with Sonchus asper in rat. Food Chem Toxicol. 2010;48(8–9):2469–76. doi:10.1016/j.fct.2010.06.016.
Guengerich FP, Churchill PF, Jung CY, Fleischer S. Target inactivation analysis applied to determination of molecular weights of rat liver proteins in the purified state and in microsomal membranes. Biochim Biophys Acta. 1987;915(2):246–53.
Lowry OH, Rosenbrough NG, Farr AL, Randall RJ. Protein measurements with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–75.
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–78.
Bradley PP, Priebat DA, Christensen RD, Rothstein GR. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78(3):206–9.
Koop DR. Hydroxylation of p-nitrophenol by rabbit ethanol-inducible cytochrome P-450 isozyme 3a. Mol Pharmacol. 1986;29(4):399–404.
Metwally NS, Hamed MA, Ahmed SA. Association between efficiency of certain medicinal plants and severity of renal disorders in rats. Int J Pharm Pharm Sci. 2012;4(3):432–8.
Hismiogullari AA, Hismiogullari SE, Karaca O, Sunay FB, Paksoy S, Can M, et al. The protective effect of curcumin administration on carbon tetrachloride (CCl4)-induced nephrotoxicity in rats. Pharmacol Rep. 2015;67(3):410–6. doi:10.1016/j.pharep.2014.10.021 (Epub 2014 Nov 11).
Aldini G, Orioli M, Rossoni G, Savi F, Braidotti P, Vistoli G, et al. The carbonyl scavenger carnosine ameliorates dyslipidaemia and renal function in Zucker obese rats. J Cell Mol Med. 2011;15(6):1339–54. doi:10.1111/j.1582-4934.2010.01101.x.
Yan SL, Wu ST, Yin MC, Chen HT, Chen HC. Protective effects from carnosine and histidine on acetaminophen-induced liver injury. J Food Sci. 2009;74(8):H259–65. doi:10.1111/j.1750-3841.2009.01330.x.
Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta. 2003;329:23–38.
Baykara B, Micili SC, Tugyan K, Tekmen I, Bagriyanik H, Sonmez U, et al. The protective effects of carnosine in alcohol-induced hepatic injury in rats. Toxicol Ind Health. 2014;30(1):25–32. doi:10.1177/0748233712446722.
Yilmaz-Ozden T, Can A, Karatug A, Pala-Kara Z, Okyar A, Bolkent S. Carbon tetrachloride-induced kidney damage and protective effect of Amaranthus lividus L. in rats. Toxicol Ind Health. 2014;0748233714555390. [Epub ahead of print].
Chen X, Ying X, Zhang W, Chen Y, Shi C, Hou Y, et al. The hepatoprotective effect of fraxetin on carbon tetrachloride induced hepatic fibrosis by antioxidative activities in rats. Int Immunopharmacol. 2013;17(3):543–7. doi:10.1016/j.intimp.2013.08.006.
Shimizu H, Bolati D, Adijiang A, Muteliefu G, Enomoto A, Nishijima F, et al. NF-kappaB plays an important role in indoxyl sulfate-induced cellular senescence, fibrotic gene expression, and inhibition of proliferation in proximal tubular cells. Am J Physiol Cell Physiol. 2011;301:C1201–12. doi:10.1152/ajpcell.00471.2010.
Janssen B, Hohenadel D, Brinkkoetter P, Peters V, Rind N, Fischer C, et al. Carnosine as a protective factor in diabetic nephropathy: association with a leucine repeat of the carnosinase gene CNDP1. Diabetes. 2005;54(8):2320–7.
Yao QY, Xu BL, Wang JY, Liu HC, Zhang SC, Tu CT. Inhibition by curcumin of multiple sites of the transforming growth factor-beta1 signalling pathway ameliorates the progression of liver fibrosis induced by carbon tetrachloride in rats. BMC Complement Altern Med. 2012;12:156. doi:10.1186/1472-6882-12-156.
Hamed MA, Ali SA, El-Rigal NS. Therapeutic potential of ginger against renal injury induced by carbon tetrachloride in rats. Sci World J. 2012;2012:840421. doi:10.1100/2012/840421 (Epub 2012 Apr 1).
Yay A, Akkuş D, Yapıslar H, Balcıoglu E, Sonmez MF, Ozdamar S. Antioxidant effect of carnosine treatment on renal oxidative stress in streptozotocin-induced diabetic rats. Biotech Histochem. 2014;89(8):552–7. doi:10.3109/10520295.2014.913811.
Acknowledgments
The authors would like to express their deepest thanks to the technicians in Biochemistry department and Pharmacology and Toxicology department, the technical assistance in histopathological examination and the valuable comments of Dr. Darin A. Ali, Lecturer of Histopathology, Faculty of Medicine, Tanta University, Egypt.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Keshk, W.A., Katary, M.A. Transforming Growth Factor-β1/Smad3 Signaling and Redox Status in Experimentally Induced Nephrotoxicity: Impact of Carnosine. Ind J Clin Biochem 32, 19–25 (2017). https://doi.org/10.1007/s12291-016-0564-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-016-0564-y