Abstract
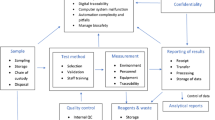
Laboratories have a major impact on patient safety as 80–90 % of all the diagnosis are made on the basis of laboratory tests. Laboratory errors have a reported frequency of 0.012–0.6 % of all test results. Patient safety is a managerial issue which can be enhanced by implementing active system to identify and monitor quality failures. This can be facilitated by reactive method which includes incident reporting followed by root cause analysis. This leads to identification and correction of weaknesses in policies and procedures in the system. Another way is proactive method like Failure Mode and Effect Analysis. In this focus is on entire examination process, anticipating major adverse events and pre-emptively prevent them from occurring. It is used for prospective risk analysis of high-risk processes to reduce the chance of errors in the laboratory and other patient care areas.
Similar content being viewed by others
References
O’Kaine M. The reporting, classification and grading of quality failures in the medical laboratory. Clin Chem Acta. 2009;404:28–31.
Choozza ML, Ponzetti C. FMEA: a model for reducing medical errors. Clin Chem Acta. 2009;404:75–8.
Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med. 1991;7(324):377–84.
Reason JT, Carthey J, de Leval MR. Diagnosing ‘vulnerable system syndromes’: an essential prerequisite to effective risk management. Qual Health Care. 2001;10 Suppl 2:ii21–5.
Reason J. Safety in the operating theatre—part 2: human error and organisational failure. Qual Saf Health Care. 2005;14:56–61.
Reason J. Combating omission errors through task analysis and good reminders. Qual Saf Health Care. 2002;11:40–4.
Carthey J, de Leval MR, Reason JT. Institutional resilience in health care systems. Qual Health Care. 2001;10:29–32.
Howanitz PJ. Errors in laboratory medicine. Arch Pathol Lab Med. 2005;129:1252–61.
Plebani M, Carraro P. Mistakes in a stat laboratory: types and frequency. Clin Chem. 1997;43:1348–51.
Witte DL, VanNess SA, Angstadt DS, Pannell BJ. Errors, mistakes, blunders, outliers or unacceptable results: how many? Clin Chem. 1997;43:1352–6.
Goldschmidt HMJ, Lent RW. Gross errors and work flow analysis in the clinical laboratory. Klin Biochem Metab. 1995;3:131–40.
O’Kane MJ, Lynch PLM, McGowan N. The development of a system for the reporting, classification and grading of quality failures in the clinical biochemistry laboratory. Ann Clin Biochem. 2008;45:129–34.
International Organization for Standardization. ISO/PDTS 22367. Medical laboratories: reducing error through risk management and continual improvement: complementary element. Geneva: ISO; 2005.
Woodhouse S, Burney B, Costie K. To err is human: improving patient safety through failure mode and effect analysis. Clin Leadersh Manag Rev. 2004;18:32–6.
Capunzo M, Cavallo P, Boccia G, Brunetti L, Pizzuti S. A FMEA clinical laboratory case study: how to make problems and improvements measurable. Clin Leadrersh Manag Rev. 2004;18:37–41.
International Organization for Standardization. ISO/PDTS 22367:2008. Medical laboratories—reduction of error through risk management and continual improvement. Geneva: ISO; 2008.
Joint Commission on Accreditation of Health Care Organizations. Performance improvement. 2002 Comprehensive accreditation manual for hospitals. Oakbrook Terrace: Joint Commission on Accreditation of Health Care Organizations; 2001. p. 200–1.
Cohen MR, Sanders J, Davis NM. Failure mode and effects analysis: a novel approach to avoiding dangerous medication errors and accidents. Hosp Pharm. 1994;29:319–30.
Joint Commission Resources. Failure mode and effects analysis in health care: proactive risk reduction. Oakbrook Terrace: Joint Commission Resources; 2002.
Oldenhof MT, van Leeuwen JF, Nauta MJ, de Kaste D, et al. Consistency of FMEA used in the validation of analytical procedures. J Pharm Biomed Anal. 2001;4(3):592–5.
Bonini P, Plebani M, Ceriotti F, Rubboli F. Errors in laboratory medicine. Clin Chem. 2002;48:691–8.
Southard PB, Kumar S, Southard CA. A modified Delphi methodology to conduct a failure modes effects analysis: a patient-centric effort in a clinical medical laboratories. Qual Manag Health Care. 2011;20(2):131–51.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Agarwal, R. Measurement of Errors in Clinical Laboratories. Ind J Clin Biochem 28, 227–234 (2013). https://doi.org/10.1007/s12291-013-0314-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-013-0314-3