Abstract
Immune thrombocytopenic purpura (ITP) is an autoimmune disease with possible dysregulation of the apoptotic pathways. We aimed to evaluate the possible role of some apoptotic markers (caspase 3, caspase 8 and BCL2) in the pathogenesis and course of ITP. We investigated some apoptotic markers (caspase 3, caspase 8 and BCL2) using the flow cytometry in 60 children with newly diagnosed ITP, 20 children with chemotherapy-related thrombocytopenia (CRT) and 20 healthy children. We also assessed the effects of intravenous immunoglobulin (IVIG) and methyl prednisolone therapies on the platelet apoptosis in children with newly diagnosed ITP. We demonstrated significantly higher values of caspase 3 in the newly diagnosed ITP group than control and CRT groups, and non-significantly higher values of caspase 8 in the ITP group than the healthy group. After IVIG treatment, the platelet count increased in all patients, and there was a significant decrease in caspase 3 and caspase 8 levels while BCL2 level increased. Regarding methylprednisolone treatment, there was a significant decrease in BCL2 and caspase 8 levels while caspase 3 levels did not significantly decrease. There is a possible role of the caspase dependent cell death pathway of the platelets in the occurrence of newly diagnosed ITP. There is heterogeneity in the apoptotic changes of newly diagnosed ITP children who received IVIG versus those who received methylprednisolone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immune thrombocytopenia (ITP) is an autoimmune disease with an incidence ranging from 3 to 5 per 100,000 individuals. In the pediatric age group, ITP is mostly acute self-limiting and induced by viral infections, while in adults the disorder is usually chronic. The disease could be asymptomatic or manifested by easy bruising, bleeding tendency, or blood extravasation from capillaries into the mucous membranes and skin [1, 2].
ITP pathogenesis involves both increased peripheral platelet destruction and suppression of the platelet production [1]. Increased platelets destruction is attributed to immunoglobulin G autoantibodies against platelet membrane glycoproteins. Abnormal T-cells activity represents the stimulus for the autoantibody production [3]. Coating of platelets with IgG renders them more prone to opsonization and phagocytosis by the reticuloendothelial system [4].
Apoptosis is considered the main pathophysiological process that controls the cell life span and removal of the infected or damaged nucleated cells. Although platelets are anucleate cells, they undergo events similar to apoptosis [5, 6]. There are two main apoptotic pathways that could regulate the programmed platelet death: the mitochondrial or intrinsic pathway and the death receptor or extrinsic pathway [7]. The latter is initiated by the ligation of death receptors, belonging to the tumor necrosis factor receptor superfamily, that activate the initiator caspase (procaspase 8) with subsequent activation of the effector caspases by caspase 8, such as caspase 3, 6 and 7 [8]. While the intrinsic apoptosis pathway is regulated by members of the B-cell lymphoma protein 2 family (BCL2), which contain both pro-survival and pro-death proteins that could induce changes in the mitochondrial outer membrane with subsequent efflux of the cytochrome c and other apoptogenic proteins into the cytoplasm. Once released, cytochrome c assembles with the initiator caspase (procaspase 9) and apoptotic protease-activating factor 1 to form the apoptosome, which activates caspase 9 and downstream effector caspases [8].
Murine ITP studies have shown that antiplatelet antibodies could induce apoptotic-like processes, and that the intravenous immunoglobulin (IVIG) treatment could decrease these events [9, 10]. Concerning human ITP, platelet apoptosis was suggested in children with newly diagnosed ITP in the form of activated caspase 3, 8 and 9, which were suppressed by the IVIG infusion [11]. Moreover, another study revealed that platelets from adult patients with chronic ITP expressed higher phosphatidylserine exposure associated with dysfunction of the dendritic cells, although other platelet apoptosis markers could not be demonstrated in this study [12]. A more recent study concerning adults with ITP also confirmed the relevance of platelet apoptosis in ITP [13].
The initial lines of ITP treatment include steroids to suppress the immune system and limit platelets destruction. The dose and mode of administration are determined by the platelet count and presence of active bleeding [14]. IVIG is another drug of choice as it leads to increased platelet counts, besides blockage of the reticuloendothelial system via Fc-receptors and it interferes with the apoptotic processes in platelets [14,15,16,17].
To our knowledge, few studies were conducted about apoptosis markers of newly diagnosed ITP in the pediatric age group in humans [11, 18]. Most of the previous studies, concerning apoptosis in ITP, were conducted in animal models or human adults [9, 10, 12, 13].
We conducted our preliminary study to investigate the possible role of the apoptotic markers (caspase 3, caspase 8 and BCL2) in the pathogenesis and course of newly diagnosed ITP, being markers for both apoptosis pathways (caspase 3 and caspase 8 for the extrinsic pathway while BCL2 for the intrinsic pathway). We also evaluated the effects of the treatment on the apoptotic markers of the newly diagnosed ITP children.
Materials and Methods
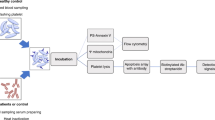
Study Design and Participants
An observational prospective preliminary study was carried out in a university-affiliated hospital from June 2019 to May 2021. We enrolled 60 patients with newly diagnosed primary ITP. We also recruited 20 healthy control children from the general outpatient clinics of same hospital while attending for minor complaints or for following up growth and development. In addition, to control effects due to thrombocytopenia itself, 20 children, with chemotherapy-related thrombocytopenia (CRT), were recruited [11]. The platelet count used to define ITP was below 100 × 109/L [19]. We excluded chronic ITP cases, adult ITP cases, secondary ITP and inherited thrombocytopenia. Patients with comorbidities including diabetes mellitus, uncontrolled hypertension, kidney and liver dysfunction, hepatitis C and B viral infections, human immunodeficiency virus and those who had treatment with corticosteroids during the previous six months were also excluded. Patients who developed serious side effects such as anaphylaxis or uncontrolled hypertension during therapy were also ruled out from the study.
Data Collection
Data were retrieved from patients’ files including the age, gender, prodrome (e.g. history of preceding fever), clinical signs (such as active bleeding, lymphadenopathy and palpable spleen / liver), preceding drug intake, laboratory investigations (complete blood count, antinuclear antibodies, anti-ds DNA, erythrocyte sedimentation rate, complement 3, and bone marrow aspiration if done) and treatment lines. We also retrieved data about infection markers of human immunodeficiency virus, hepatitis C & B viruses, tuberculosis and helicobacter pylori.
Assessment of Serum Caspase 3, Caspase 8 and BCL2 by the Flow Cytometry
Patients with newly diagnosed ITP were sampled for flow cytometric analyses at the time of diagnosis prior to treatment and 12–24 h after the last treatment dose (prior to discharge from hospital) [11, 18]. Samples from both control and CRT groups were withdrawn once at the time of enrollment. The apoptosis markers were assessed using the FACS Aria III flow cytometer (BD Biosciences, San Jose, USA). The whole blood lysis method was used, and appropriate combinations of monoclonal antibodies (Becton Dickinson Quanti BRITE products) were used. The used monoclonal antibodies were fluorochrome inhibitors of caspases FITC (Rabbit Anti-Active Caspase-3) and Rbm Ab to caspase 8 (E6) and BCL2 oncoprotein /FITC clone 124 [20,21,22,23,24].
Randomization of the Newly Diagnosed ITP Group According to the Type of Treatment
Sixty consecutive patients with newly diagnosed ITP were randomly assigned into two subgroups: 32 children (18 boys and 14 girls) received IVIG and 28 children (14 boys and 14 girls) received methyl prednisolone using a simple randomization technique with opaque sealed envelopes. Researchers who assessed outcomes were blinded to the given treatment.
Statistical Analysis
Data analysis was carried out by SPSS program, version 26. Qualitative variables were expressed as numbers (percent). Kolmogorov-Simonov test was used to test the data normality. Normally distributed data were presented as mean ± standard deviation (SD), while non-normally distributed data were presented as median and range (minimum–maximum). Student's t-tests were used for group comparison. P values equal to or less than 0.05 were considered significant.
Results
Demographic Data
One hundred children were included and divided into: (a) 60 children with newly diagnosed ITP [28 boys (46.7%) and 32 girls (53.3%)]; their ages ranged between 1–13 years (median = 4 years). (b) 20 children with CRT [6 boys (30%) and 14 girls (70%)]; their ages ranged between 4–9 years (median = 5 years). (c) 20 healthy control children [4 boys (20%) and 16 girls (80%)]; their age ranged between 4–10 years (median = 6 years) (Table 1). No statistically significant differences existed between the study groups regarding the age (P = 0.29) and the gender (P = 0.27). No mortality or serious side effects were reported throughout the study period in patient groups.
Initial Values of the Apoptotic Markers of the Study Groups
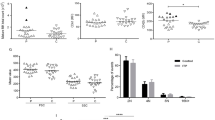
In the newly diagnosed ITP group, there were significantly higher values of caspase 3 when compared with control and CRT groups (P = 0.001 for each). As regards caspase 8, there was insignificant difference between the ITP group and the CRT group (P = 0.69) while there were significant differences between the ITP group and the control group (P < 0.001) and between the control and CRT groups (P < 0.001). As regards BCL2, there was a significant higher value in the newly diagnosed ITP group than the control group (P = 0.008), while there were insignificant differences between the CRT group and ITP and control groups (Table 2 and Fig. 1).
The Overall Effect of the Treatment on the Apoptotic Markers of the Newly Diagnosed ITP Group
The newly diagnosed ITP group showed significantly higher values of caspase 3, caspase 8 and BCL2 in the pretreatment state than the post treatment state (P = 0.01, < 0.001 and 0.034, respectively) (Table 3).
The Individual Effects of IVIG or Methyl Prednisolone on the Apoptotic Markers of the Newly Diagnosed ITP Group
In the pretreatment state, there were no significant differences in caspase 3, caspase 8 and BCL2 levels between both subgroups (IVIG and methyl prednisolone). Whereas, there were significant lower values of caspase 3 and caspase 8 levels after receiving IVIG than methylprednisolone (P = 0.017 and 0.003, respectively). Whereas, there was a significant lower level of BCL2 after treatment in the methylprednisolone group compared with IVIG group (P = 0.036, Table 4).
Apoptotic Markers Values Pre and Post Treatment in IVIG and Methylprednisolone Groups
Regarding the IVIG treatment, there was a significant reduction in caspase 3 (P = 0.002) and caspase 8 (P < 0.001) levels after treatment and a significant increase in the BCL2 level after the treatment (P < 0.001). While with the methylprednisolone treatment, caspase 8 and BCL2 levels decreased after the treatment (P < 0.001). Meanwhile, there were insignificant changes regarding caspase 3 (P = 0.314) (Fig. 2).
a–c Significant reductions in caspase 3 (P = 0.002), caspase 8 (P < 0.001) and significant increase in BCL2 (P < 0.001) in the newly diagnosed ITP patients treated with IVIG. d–f Significant reductions in caspase 8 and BCL2 (P < 0.001), with non-significant change in caspase 3 levels in the newly diagnosed ITP patients treated with methylprednisolone (P = 0.314)
Discussion
ITP is an autoimmune disorder with possible involvement of the apoptosis pathways [7].The present study revealed higher levels of all apoptotic markers in the newly diagnosed ITP cases, denoting involvement of both apoptosis pathways. This is in agreement with Speer and Schmugge who reported that platelets undergo apoptotic-like events through translocation and activation of BCL2 family members [25]. In contrary, there is less pronounced caspase activation and resistance to platelet apoptosis in chronic ITP [26, 27].
Newly diagnosed ITP patients, after IVIG treatment, had considerable decrease in caspase 3 & caspase 8 while BCL2 increased. The clinical presentation of ITP receded and the platelet count increased. Meanwhile, after treatment with methylprednisolone, there was significant decrease in caspase 8 and BCL2 while caspase 3 did not significantly decrease.
Regarding BCL2, there was a significant decrease with the methyl prednisolone treatment and a significant increase with the IVIG treatment. These findings indicate heterogeneous changes in the apoptotic ITP pathways, based upon the used treatment. IVIG and methylprednisolone possibly affected platelet apoptosis pathways in different ways. Recognition of these apoptotic changes may open the gate for better understanding of the mechanism of action of these therapies. These apoptosis markers could be possibly used as follow-up markers for the treatment response. But, further extended large scale studies with more apoptosis markers are still needed to confirm our results.
These finding were similar to a previous study that reported a decrease in the proportion of platelets with caspase 8 & caspase 3 after the IVIG treatment [11]. Leytin et al. [9] also confirmed that IVIG prevents downstream apoptotic events and caspase 3 activation. Speer and Schmugge [25] also observed activation of caspase 3 in 40% of the platelets in primary ITP and decrease to 10% after the IVIG therapy. Several studies approved that the apoptotic platelets events such as activation of caspase 3, 8 and 9 increase specifically in ITP but not in CRT and decrease after the IVIG treatment [11, 18, 25].
The strengths of our study included the prospective design. Understanding the effects of two well-known lines of treatment on the apoptotic markers of the newly diagnosed ITP children represents a new add to literature. Previous human reports focused mainly on the effects of the IVIG therapy on apoptosis [11]. However, the current study had some limitations, being a single center study with a relatively small sample size and had limited studied apoptotic markers and a short follow-up period.
Conclusion
There is a possible role of the caspase dependent cell death pathway of the platelets in the occurrence of newly diagnosed ITP in children. There are heterogeneous changes in the apoptotic markers of newly diagnosed ITP, based upon the used therapy. Levels of caspase decrease when children received IVIG or methylprednisolone treatment but more pronounced with IVIG, while the BCL2 level increases with the IVIG therapy and decrease with methylprednisolone. In the future studies, we recommend extended large scale multicenter studies with more apoptosis markers to generalize our results.
Data, Materials and/or Code availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Abadi U, Yarchovsky-Dolberg O, Ellis MH (2015) Immune thrombocytopenia: recent progress in pathophysiology and treatment. Clin Appl Thromb Hemost 21(5):397–404
Fogarty PF, Segal JB (2007) The epidemiology of immune thrombocytopenic purpura. Curr Opin Hematol 14(5):515–519
Stasi R, Cooper N, Del Poeta G, Stipa E, Laura Evangelista M, Abruzzese E et al (2008) Analysis of regulatory T-cell changes in patients with idiopathic thrombocytopenic purpura receiving B cell-depleting therapy with rituximab. Blood 112(4):1147–1150
Schwartz RS (2007) Immune thrombocytopenic purpura–from agony to agonist. N Engl J Med 357(22):2299–2301
Böing AN, Hau CM, Sturk A, Nieuwland R (2008) Platelet microparticles contain active caspase 3. Platelets 19(2):96–103
Lopez J, Salido G, Pariente J, Rosado J (2008) Thrombin induces activation and translocation of Bid, Bax and Bak to the mitochondria in human platelets. J Thromb Haemost 6(10):1780–1788
Igney FH, Krammer PH (2002) Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer 2(4):277–288
Youle RJ, Strasser A (2008) The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9(1):47–59
Leytin V, Mykhaylov S, Starkey AF, Allen DJ, Lau H, Ni H et al (2006) Intravenous immunoglobulin inhibits anti-glycoprotein IIb-induced platelet apoptosis in a murine model of immune thrombocytopenia. Br J Haematol 133(1):78–82
Song S, Crow AR, Freedman J, Lazarus AH (2003) Monoclonal IgG can ameliorate immune thrombocytopenia in a murine model of ITP: an alternative to IVIG. Blood 101(9):3708–3713
Winkler J, Kroiss S, Rand ML, Azzouzi I, Annie Bang KW, Speer O et al (2012) Platelet apoptosis in paediatric immune thrombocytopenia is ameliorated by intravenous immunoglobulin. Br J Haematol 156(4):508–515
Catani L (2006) Dendritic cells of immune thrombocytopenic purpura (ITP) show increased capacity to present apoptotic platelets to T lymphocytes. Exp Hematol 34(7):879–887
Goette NP, Glembotsky AC, Lev PR, Grodzielski M, Contrufo G, Pierdominici MS et al (2016) Platelet apoptosis in adult immune thrombocytopenia: insights into the mechanism of damage triggered by auto-antibodies. PLoS ONE 11(8):e0160563
Cines DB, Bussel JB (2005) How I treat idiopathic thrombocytopenic purpura (ITP). Blood 106(7):2244–2251
Stevens W, Koene H, Zwaginga JJ, Vreugdenhil G (2006) Chronic idiopathic thrombocytopenic purpura: present strategy, guidelines and new insights. Neth J Med 64(10):356–363
Bussel JB, Cheng G, Saleh MN, Psaila B, Kovaleva L, Meddeb B et al (2007) Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med 357(22):2237–2247
Kuter DJ, Rummel M, Boccia R, Macik BG, Pabinger I, Selleslag D et al (2010) Romiplostim or standard of care in patients with immune thrombocytopenia. N Engl J Med 363(20):1889–1899
Winkler J, Kroiss S, Rand ML, Schmugge M, Speer O (2010) Activation of caspases-8 and -9 Occurs in platelets from children with immune, but not chemotherapy-related, thrombocytopenia. Blood 116(21):3700
Yahia S, Wahba Y, El-Gilany AH, Abdelmabood S, El-Hadidy MA, Darwish A et al (2019) Psychiatric disorders and quality of life in Egyptian patients with chronic immune thrombocytopenic purpura: a single center study. Indian J Hematol Blood Transfus 35(2):347–351
Bossuyt X, Marti GE, Fleisher TA (1997) Comparative analysis of whole blood lysis methods for flow cytometry. Cytometry 30(3):124–133
Sirchia G, Pizzi C, Scalamogna M (1972) A simple procedure for human lymphocyte isolation from peripheral blood. Tissue Antigens 2(2):139–140
Darke C, Dyer PA (1993) Clinical HLA typing by cytotoxicity. In: Dyer PA, Middleton D (eds) Histocompatibility testing: a practical approach. IRL Press, Oxford, pp 51–80
Brown M, Wittwer C (2000) Flow cytometry: principles and clinical applications in hematology. Clin Chem 46(8 Pt 2):1221–1229
Mitwalli M, Wahba Y, Shaltout A, Gouida M (2018) Lymphocyte subgroups and recurrent infections in children with Down syndrome—a prospective case control study. Cent Eur J Immunol 43(3):248–254
Speer O, Schmugge M (2010) Investigating caspases and other markers of apoptosis in ITP. Ann Hematol 89 Suppl 1(Suppl 1):45–46
Nugent D, McMillan R, Nichol JL, Slichter SJ (2009) Pathogenesis of chronic immune thrombocytopenia: increased platelet destruction and/or decreased platelet production. Br J Haematol 146(6):585–596
Wang JD, Ou TT, Wang CJ, Chang TK, Lee HJ (2010) Platelet apoptosis resistance and increased CXCR4 expression in pediatric patients with chronic immune thrombocytopenic purpura. Thromb Res 126(4):311–318
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No funds, grants, or other support was received.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Ethics Approval
All procedures involving human participants were in accordance with the ethical standards of Institutional Research Board of Mansoura University Faculty of Medicine, Mansoura, Egypt (Code number: R.20.08.1000), and the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed Consent
Written informed consents were taken from legal guardians of all study participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yahia, S., Eldars, W., Eldegla, H. et al. Cell Death Markers in Children with Immune Thrombocytopenic Purpura: A Preliminary Study. Indian J Hematol Blood Transfus 39, 635–641 (2023). https://doi.org/10.1007/s12288-023-01639-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12288-023-01639-0