Abstract
Background
HER2-low breast cancer (BC) is proposed to be a special population of patients with an immunohistochemistry (IHC) score of 1 + or 2 + and non-amplified in situ hybridization (ISH) results. The role and prognostic impact of HER2-low BC is still controversial. This meta-analysis aims to explore the prognostic difference between of HER2-low and HER2-zero characteristic in BC patients.
Methods
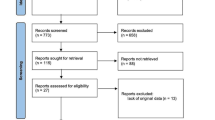
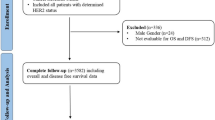
A meta-analysis was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and eligible studies were search in PubMed, Web of Science and EMBASE databases. Quality assessment of included studies were performed by Quality in Prognostic Studies (QUIPS) tool. Hazard ratios (HRs) and corresponding 95% confidence interval (CI) for overall survival (OS) and disease-free survival (DFS) were pooled in a meta-analysis. Furthermore, subgroup analysis, sensitivity analysis, and analysis for publication bias were conducted.
Results
Eighteen studies comprising a total of 93,317 patients were included for meta-analysis. BC patients with HER2-low characteristic have longer OS (HRs 0.87, 95% CI 0.81–0.93, p < 0.0001) and DFS (HRs 0.82, 95% CI 0.73–0.93, p = 0.001) compared to those with HER2-zero characteristic. Subgroup analysis indicate that the source of heterogeneity may come from the hormone receptor (HR) status group. Although, the publication bias was detected, sensitivity analysis and the trim-and-fill method analysis demonstrated the stability and reliability of the results.
Conclusion
HER2-low BC patients have longer OS and DFS compared to HER2-zero BC patients, and its prognostic value is consistent among different HR status patients. Whether HER2-low breast cancer is an independent subtype of breast cancer is still a subject of ongoing research, and more studies are needed to fully understand the molecular and clinical features of this subtype.






Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author, Gaosong Wu (wugaosongtj@163.com), upon reasonable request.
Abbreviations
- BC:
-
Breast cancer
- IHC:
-
Immunohistochemistry
- ISH:
-
In situ hybridization
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- QUIPS:
-
Quality in prognostic studies
- HRs:
-
Hazard ratios
- OS:
-
Overall survival
- DFS:
-
Disease-free survival
- HER2:
-
Growth factor receptor 2
- ASCO/CAP:
-
American Society of Clinical Oncology/College of American Pathologists
- ADCs:
-
Antibody–drug conjugates
- T-DXd:
-
Trastuzumab deruxtecan
- NGS:
-
Next-generation sequencing
- N/P:
-
Negative or positive
- HL:
-
HER2-low
- HZ:
-
HER2-zero
- TNBC:
-
Triple-negative breast cancer
- FUSCC:
-
Fudan University Shanghai Cancer Center
- IDC:
-
Invasive ductal carcinomas
- ILC:
-
Invasive lobular carcinomas
- ESME:
-
Epidemiological strategy and medical economics
- ER:
-
Estrogen receptor
References
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. https://doi.org/10.3322/caac.21763.
Chung A, Cui X, Audeh W, Giuliano A. Current status of anti-human epidermal growth factor receptor 2 therapies: predicting and overcoming herceptin resistance. Clin Breast Cancer. 2013;13(4):223–32. https://doi.org/10.1016/j.clbc.2013.04.001.
Hudis CA. Trastuzumab–mechanism of action and use in clinical practice. N Engl J Med. 2007;357(1):39–51. https://doi.org/10.1056/NEJMra043186.
Baselga J, Cortés J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109–19. https://doi.org/10.1056/NEJMoa1113216.
Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol. 2018;36(20):2105–22. https://doi.org/10.1200/JCO.2018.77.8738.
Fehrenbacher L, Cecchini RS, Geyer CE Jr, et al. NSABP B-47/NRG oncology phase III randomized trial comparing adjuvant chemotherapy with or without trastuzumab in high-risk invasive breast cancer negative for HER2 by FISH and with IHC 1+ or 2. J Clin Oncol. 2020;38(5):444–53. https://doi.org/10.1200/JCO.19.01455.
Tarantino P, Hamilton E, Tolaney SM, et al. HER2-low breast cancer: pathological and clinical landscape. J Clin Oncol. 2020;38(17):1951–62. https://doi.org/10.1200/JCO.19.02488.
Modi S, Jacot W, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387(1):9–20. https://doi.org/10.1056/NEJMoa2203690.
Denkert C, Seither F, Schneeweiss A, et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 2021;22(8):1151–61. https://doi.org/10.1016/S1470-2045(21)00301-6.
Li Y, Abudureheiyimu N, Mo H, et al. In real life, low-level HER2 expression may be associated with better outcome in HER2-negative breast cancer: a study of the National Cancer Center, China. Front Oncol. 2022;11:774577. https://doi.org/10.3389/fonc.2021.774577 (Published 2022 Jan 17)
Almstedt K, Heimes AS, Kappenberg F, et al. Long-term prognostic significance of HER2-low and HER2-zero in node-negative breast cancer. Eur J Cancer. 2022;173:10–9. https://doi.org/10.1016/j.ejca.2022.06.012.
de Calbiac O, Lusque A, Mailliez A, et al. Comparison of management and outcomes in ERBB2-low vs ERBB2-zero metastatic breast cancer in France. JAMA Netw Open. 2022;5(9):e2231170. https://doi.org/10.1001/jamanetworkopen.2022.31170. (Published 2022 Sep 1)
Xu H, Han Y, Wu Y, et al. Clinicopathological characteristics and prognosis of HER2-low early-stage breast cancer: a single-institution experience. Front Oncol. 2022;12:906011. https://doi.org/10.3389/fonc.2022.906011. (Published 2022 Jun 16)
Tarantino P, Jin Q, Tayob N, et al. Prognostic and biologic significance of ERBB2-low expression in early-stage breast cancer. JAMA Oncol. 2022;8(8):1177–83. https://doi.org/10.1001/jamaoncol.2022.2286.
de Moura LL, Cesca MG, Tavares MC, et al. HER2-low status and response to neoadjuvant chemotherapy in HER2 negative early breast cancer. Breast Cancer Res Treat. 2021;190(1):155–63. https://doi.org/10.1007/s10549-021-06365-7.
Mutai R, Barkan T, Moore A, et al. Prognostic impact of HER2-low expression in hormone receptor positive early breast cancer. Breast. 2021;60:62–9. https://doi.org/10.1016/j.breast.2021.08.016.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. https://doi.org/10.1371/journal.pmed.1000100
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71. (Published 2021 Mar 29)
Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280–6. https://doi.org/10.7326/0003-4819-158-4-201302190-00009.
Rosso C, Voutsadakis IA. Characteristics, clinical differences and outcomes of breast cancer patients with negative or low HER2 expression. Clin Breast Cancer. 2022;22(4):391–7. https://doi.org/10.1016/j.clbc.2022.02.008.
Miglietta F, Griguolo G, Bottosso M, et al. Evolution of HER2-low expression from primary to recurrent breast cancer [published correction appears in NPJ Breast Cancer. 2021 Nov 24;7(1):149]. NPJ Breast Cancer. 2021;7(1):137. https://doi.org/10.1038/s41523-021-00343-4. (Published 2021 Oct 12)
Gampenrieder SP, Rinnerthaler G, Tinchon C, et al. Landscape of HER2-low metastatic breast cancer (MBC): results from the Austrian AGMT_MBC-Registry. Breast Cancer Res. 2021;23(1):112.https://doi.org/10.1186/s13058-021-01492-x. (Published 2021 Dec 14)
Gampenrieder SP, Dezentjé V, Lambertini M, et al. Influence of HER2 expression on prognosis in metastatic triple-negative breast cancer-results from an international, multicenter analysis coordinated by the AGMT Study Group [published online ahead of print, 2022 Dec 21]. ESMO Open. 2022;8(1):100747. https://doi.org/10.1016/j.esmoop.2022.100747
Jiang C, Perimbeti S, Deng L, Shapiro CL, Gandhi S. Clinical outcomes of de novo metastatic HER2-low breast cancer: a national cancer database analysis. NPJ Breast Cancer. 2022;8(1):135. https://doi.org/10.1038/s41523-022-00498-8. (Published 2022 Dec 30)
Tarantino P, Gandini S, Nicolò E, et al. Evolution of low HER2 expression between early and advanced-stage breast cancer. Eur J Cancer. 2022;163:35–43. https://doi.org/10.1016/j.ejca.2021.12.022.
Tan RSYC, Ong WS, Lee KH, et al. HER2 expression, copy number variation and survival outcomes in HER2-low non-metastatic breast cancer: an international multicentre cohort study and TCGA-METABRIC analysis. BMC Med. 2022;20(1):105. https://doi.org/10.1186/s12916-022-02284-6. (Published 2022 Mar 17)
Kang S, Lee SH, Lee HJ, et al. Pathological complete response, long-term outcomes, and recurrence patterns in HER2-low versus HER2-zero breast cancer after neoadjuvant chemotherapy. Eur J Cancer. 2022;176:30–40. https://doi.org/10.1016/j.ejca.2022.08.031.
Carlino F, Diana A, Ventriglia A, et al. HER2-low status does not affect survival outcomes of patients with metastatic breast cancer (MBC) undergoing first-line treatment with endocrine therapy plus palbociclib: results of a multicenter, retrospective cohort study. Cancers (Basel). 2022;14(20):4981.https://doi.org/10.3390/cancers14204981. (Published 2022 Oct 11)
Chen M, Chen W, Liu D, et al. Prognostic values of clinical and molecular features in HER2 low-breast cancer with hormonal receptor overexpression: features of HER2-low breast cancer. Breast Cancer. 2022;29(5):844–53. https://doi.org/10.1007/s12282-022-01364-y.
Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121(7):2750–67. https://doi.org/10.1172/JCI45014.
Liu YR, Jiang YZ, Xu XE, et al. Comprehensive transcriptome analysis identifies novel molecular subtypes and subtype-specific RNAs of triple-negative breast cancer. Breast Cancer Res. 2016;18(1):33. https://doi.org/10.1186/s13058-016-0690-8. (Published 2016 Mar 15)
Gong Y, Ji P, Yang YS, et al. Metabolic-pathway-based subtyping of triple-negative breast cancer reveals potential therapeutic targets. Cell Metab. 2021;33(1):51-64.e9. https://doi.org/10.1016/j.cmet.2020.10.012.
Trihia H, Murray S, Price K, et al. Ki-67 expression in breast carcinoma: its association with grading systems, clinical parameters, and other prognostic factors–a surrogate marker? Cancer. 2003;97(5):1321–31. https://doi.org/10.1002/cncr.11188.
Dalenc F, Lusque A, De La Motte RT, et al. Impact of lobular versus ductal histology on overall survival in metastatic breast cancer: a French retrospective multicentre cohort study. Eur J Cancer. 2022;164:70–9. https://doi.org/10.1016/j.ejca.2021.12.031.
Schettini F, Chic N, Brasó-Maristany F, et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer. 2021;7(1):1. https://doi.org/10.1038/s41523-020-00208-2. (Published 2021 Jan 4).
Zhang G, Ren C, Li C, et al. Distinct clinical and somatic mutational features of breast tumors with high-, low-, or non-expressing human epidermal growth factor receptor 2 status. BMC Med. 2022;20(1):142. https://doi.org/10.1186/s12916-022-02346-9. (Published 2022 Apr 29)
Fernandez AI, Liu M, Bellizzi A, et al. Examination of low ERBB2 protein expression in breast cancer tissue. JAMA Oncol. 2022;8(4):1–4. https://doi.org/10.1001/jamaoncol.2021.7239.
Yue M, Zhang J, Wang X, et al. Can AI-assisted microscope facilitate breast HER2 interpretation? A multi-institutional ring study. Virchows Arch. 2021;479(3):443–9. https://doi.org/10.1007/s00428-021-03154-x.
Funding
This research did not receive any specific grant.
Author information
Authors and Affiliations
Contributions
CL, QY and GW designed the study; CL, QY and LZ contributed to the writing of the draft manuscript; CL and QY performed the literature search, and extracted and analyzed the data. CL, TD, GX and JH performed the statistical analysis and revised the manuscript. CL and WC wrote the manuscript. All the authors have read and approved the final submitted manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Li, C., Yuan, Q., Deng, T. et al. Prognosis difference between HER2-low and HER2-zero breast cancer patients: a systematic review and meta-analysis. Breast Cancer 30, 965–975 (2023). https://doi.org/10.1007/s12282-023-01487-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-023-01487-w