Abstract
Background
The survival outcomes in HER2-low versus HER2-zero breast cancer (BC) after neoadjuvant chemotherapy (NACT) remain unclear. The meta-analysis was conducted to summarize current evidence about the survival outcomes in HER2-low versus HER2-zero BC.
Methods
We conducted a systematic search in PubMed and EMBASE databases to identify relevant studies.
Results
A total of 14 studies with 53,714 patients were included. Overall, 34,037 patients (63.37%) were HER2-low, and 19,677 patients (36.63%) were HER2-zero. Patients with HER2-low tumors had a significantly lower pathological complete response (pCR) rate than patients with HER2-zero tumors, regardless of the hormone receptor status. Compared with HER2-zero breast cancer, the overall survival (OS) and disease-free survival (DFS) of HER2-low BC were longer in the overall cohort (HR = 0.72; 95% CI = 0.61–0.85; P < 0.0001; HR = 0.83; 95% CI = 0.75–0.92; P = 0.0002); however, no differences were observed in terms of OS and DFS between HER2-low and HER2-zero BC in the HR-negative group. In the HR-positive group, HER2-low status had no significant impact on OS, while significantly associated with increased DFS (HR = 0.85; 95% CI = 0.76–0.96; P = 0.007).
Conclusion
These results suggest that although HER2-low BC has a poor response to NACT, it is correlated with favorable OS and DFS after NACT in the overall cohort as well as longer DFS in the HR-positive group.
Similar content being viewed by others
Introduction
Human epidermal growth factor receptor 2 (HER2) is a receptor for transmembrane tyrosine kinases, which is directly related to the aggressive growth of BC and is an important target for BC treatment [1]. Trastuzumab is the first approved monoclonal antibody against HER2, which can target the extracellular domain of HER2 protein. H0648g, BCIRG 007, and other studies showed that trastuzumab combined with chemotherapy significantly prolonged the survival time of BC patients with HER2 overexpression (IHC 3 + or IHC 2 + with ISH positivity) [2, 3]. Studies such as NSABP B-47/NRG confirmed that patients with low or moderate HER2 expression (IHC 1+, or 2+/ISH negative) cannot benefit from traditional targeted drugs [4, 5]. Therefore, the HER2 status has always been divided into two categories: HER2 low or moderate expression and HER2 zero expression are classified as HER2 negative (IHC 0, 1+, or 2+/ISH negative), while HER2 overexpression (IHC 3 + or IHC 2 + with ISH positive) is classified as HER2 positive [6]. However, the recent development of novel antibody-drug conjugates (ADCs) has significantly improved the prognosis of BC patients with low or moderate HER2 expression (1+, or 2+/ISH negative), thus leading to the concept of “HER2-low breast cancer” [7, 8].
Currently, most studies define BC with low or moderate HER2 expression (IHC 1+, or 2+/ISH negative) as HER2-low BC [9,10,11,12]. Based on the fact that HER2-low BC has low or moderate HER2 expression and can benefit from new targeted drugs, some scholars proposed that HER2-low BC is different from HER2-zero, that is, different from luminal BC or triple-negative BC, and may be an independent subtype. This has aroused strong interest among researchers. It is currently known that HER2-low BC has a large population, accounting for approximately 40–50% of breast cancers [13]. The biological, clinicopathological, and prognostic differences between HER2-low and HER2-zero breast cancers have been reported [9, 10, 14, 15]. However, HER2-low BC has not yet been established as an independent subtype. Although novel targeted drugs have brought benefits to HER2-low BC, they have not yet been approved for front-line treatment of non-metastatic HER2-low BC. Chemotherapy remains one of the most important treatments for non-metastatic HER2-low BC, especially for HER2-low BC patients with HR-negative or HR-positive who are resistant to endocrine therapy. Exploring the differences in chemotherapy sensitivity and prognosis between HER2-low BC and HER2-zero BC can help us further discover the differences between HER2-low BC and HER2-zero BC, and also help us better understand the clinicopathological characteristics of HER2-low BC and its sensitivity to chemotherapy, to provide a basis for the later formulation of HER2-low BC treatment plan. However, multiple studies have reached different conclusions about the effects of HER2-low and HER2-zero on the response and prognosis of neoadjuvant chemotherapy [11, 16,17,18,19,20]. Given the conflicting conclusions, we conducted this meta-analysis to compare the survival outcomes in HER2-low versus HER2-zero BC after neoadjuvant chemotherapy.
Materials and methods
Search strategy
This meta-analysis was conducted strictly following the PRISMA 2020 statement [21]. The PRISMA checklist is shown in Additional File 1. We performed a systematic literature search in the PubMed and Embase databases for studies published by October, 2023. Keywords used were; (“breast cancer, OR breast neoplasm” AND “HER2-low”). Any geographical region or language was accepted. The detailed reproducible search strategy for each of the databases is shown in Additional File 2.
Inclusion and exclusion criteria
The published studies were to meet the following inclusion criteria; (1) BC patients diagnosed with HER2-low or HER2-zero; (2) patients treated with NACT and surgery; (3) HER2-low was defined as HER2 IHC 1 + or 2+/ISH negative. (4) the study must have reported the pCR rate and the survival outcomes in terms of OS and/or DFS; (5) the study compared the survival outcomes between HER2-low and HER2-zero. Exclusion criteria were as follows; (1) review articles, letters to the editor, comments, editorials, and case reports; (2) patients with metastatic disease or other malignant tumors; (3) Lacking data on clinical outcome that could be used to calculate the HRs and 95% CIs.
Study selection and data extraction
We selected the studies according to the search strategy and inclusion and exclusion criteria. The standardized data extraction form was used to extract the relevant information such as the first author’s name, year of publication, study design, nationality and the number of patients, the median age of participants, the tumor stage and histology, Nottingham grade, local treatment, median follow-up time, survival outcomes.
Quality assessment
The study quality was assessed based on eight items from the non-randomized experimental research-MINORS scale [22], Each item was scored as 0 (not reported), 1 (inadequately reported), or 2 (adequately reported). we only retained studies with scores of 8 or more, which were rated as high-quality (See Supplementary Table 2, Additional File 3).
Summary measures and statistical analysis
We used RevMan version 5.3 (RevMan, version 5.3 for Windows; Cochrane Collaboration, Oxford, UK) for Meta-analysis. The hazard ratios (HRs) were extracted from published.
data or Kaplan-Meier survival curves. Statistical heterogeneity was assessed by Chi-squared and I2. When I2 > 50%, the test of heterogeneity was significant, thus, the random-effects model was used; otherwise, the fixed-effects model was used [23]. Funnel plot and Begg’s test were used to assess the potential publication bias [24]. They were performed with the Stata Version 11.0 (Stata Corporation, College Station, TX, USA). All tests were two-sided. P < 0.05 was considered statistically significant.
Results
Study selection and characteristics
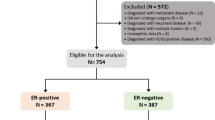
A total of 946 articles were identified. 773 articles were retained after removing duplicates. After reviewing the title and abstract, 658 articles irrelevant to this study were also removed. 13 articles were excluded after the full text was reviewed according to the inclusion and exclusion criteria. In the end, 14 studies with 53,714 patients who meet the criteria were included [11, 13, 16,17,18,19,20, 25,26,27,28,29,30,31]. As shown in Fig. 1.
All the included studies were retrospective cohort studies published in 2021 to 2023. In the studies, 34,037 patients (63.37%) were HER2-low, and 19,677 patients (36.63%) were HER2-zero. 10 studies reported that 21,190 (67.11%) of 31,574 patients with HER2-low were HR-positive, compared with 8753 (46.98%) of 18,631 patients with HER2-zero. The information on characteristics is shown in Tables 1 and 2.
Pathologic complete response
8324 out of 31,576 patients with HER2-low achieved pCR, while 6369 out of 18,631 patients with HER2-zero achieved pCR. HER2-low patients had a significantly lower pCR rate than HER2-zero patients (26.36% VS. 34.18%, OR = 0.63; 95% CI = 0.55–0.71; P < 0.00001). Heterogeneity was detected among these data (I 2 = 52%, P = 0.02). According to hormone receptor status, the pCR rate of HER2-low patients was also lower than that of HER2-zero patients in HR-positive group (16.48% vs. 19.22.00%, OR = 0.82; 95% CI = 0.77–0.87; P < 0.00001) and HR-negative group(45.77% vs. 47.16%, OR = 0.93; 95% CI = 0.87–0.98; P = 0.006). There was no heterogeneity in the two subgroups. As shown in Fig. 2.
Overall survival
Eleven studies involving 49,763 people reported OS. After a median follow-up of 46.6 months, HER2-low patients showed a longer OS than HER2-zero (HR = 0.72; 95% CI = 0.61–0.85; P < 0.0001). In the subgroup analysis, there was no significant difference in OS between HER2-low and HER2-zero patients in the HR-positive group (HR = 0.83; 95% CI = 0.68–1.01; P = 0.07) and HR-negative group (HR = 0.88; 95% CI = 0.70–1.10; P = 0.27). Significant heterogeneity existed among the studies. As shown in Fig. 3.
Disease-free survival
Nine studies involving 7569 people reported DFS. After a median follow-up of 35.75 months, the DFS of HER2-low patients is significantly better than that of HER2-zero patients (HR = 0.83; 95% CI = 0.75–0.92; P = 0.0002). According to hormone receptor status, this survival trend was also true in the subgroups of patients with HR-positive tumors (HR = 0.85; 95% CI = 0.76–0.96; P = 0.007). However, no survival difference was seen between them in HR-negative tumors (HR = 0.95; 95% CI = 0.71–1.29; P = 0.76). Heterogeneity only exists in the HR-negative group. As shown in Fig. 4.
The funnel plots and Begg’s test were used to detect the publication bias ( Fig. 5). All P values were > 0.05 ( See Supplementary Table 3, Additional File 4), suggesting no potential publication bias was found in the pCR rate, OS and DFS.
Funnel plot of HER2-low breast cancer vs. HER2-zero breast cancer. OR for the pCR rate: overall cohort (a), HR-positive group(b), HR-negative group(c); HR for OS: overall cohort (d), HR-positive group(e), HR-negative group(f); HR for DFS: overall cohort (g), HR-positive group(h), HR-negative group(i)
Discussion
HER2-low BC is now increasingly considered a distinct subtype. We analyzed the outcomes of HER2-low BC through 34,037 patients in 14 studies. A lower rate of pCR was observed in the patients with HER2-low versus those with HER2-zero, regardless of the hormone receptor status. HER2-low patients had superior OS and DFS compared to HER2-zero patients in the overall group. According to hormone receptor status, HER2-low patients led to a better DFS in the HR-positive group, while no significant survival difference was seen between them in other groups.
Previous studies reported that the proportion of HER2-low in HER2-negative ranged from 33.3 to 72.1% [13, 32,33,34]. Our study shows that the proportion is 63.37%. The difference in proportion between different studies may be due to the limitations of current detection and interpretation of HER2 expression. At present, the difficulties and inconsistencies mainly focus on the interpretation of 0 and 1+. According to the American Society of Clinical Oncology(ASCO) guideline: IHC 0: no staining or ≤ 10% of cells with incomplete membrane staining that is faint / barely perceived. IHC 1+: >10% of cells with incomplete membrane staining that is faint/barely perceptible [6]. Patients with 1 + have weak staining and need to be observed more closely. The interpretation of human eyes is subjective and inconsistent. Previously, the treatment of HER2 (1+) was the same as HER2-zero, so the pathologist himself may not pay much attention to the distinction between HER2 immunohistochemistry 0 and 1+. Secondly, in the detection process, the detection antibodies and platforms of each pathology department are different, which may also lead to differences in the determination. In 2023, ASCO guidelines specifically proposed 5 recommendations to distinguish IHC 1 + results from 0 in the update of HER2 testing guidelines. For example, examining HER2 IHC at high power (40×) when discriminating 0 from 1 + staining and considering a second pathologist review when results are close to the 0 versus 1 + interpretive threshold, and so on [35]. Various studies on how to achieve accurate detection of HER2 expression are also being tried. For example, droplet microfluidic technology [36], analysis of HER2 messenger RNA levels [37, 38], and digital pathology achieved through artificial intelligence [39, 40].
The results of this study showed that Luminal BC accounted for the majority of HER2-low patients, and the pCR rate of HER2-low patients was lower than that of HER2-zero. This is consistent with previous research. Schettini et al. studied the expression of PAM50 and individual gene expression in 1,320 patients (35.8%) and found that about 65% of HER2-low patients were HR-positive compared with the HER2-zero group. In the overall cohort, the expression of relative proliferation-related genes is significantly down-regulated and the expression of luminal-related genes is up-regulated in HER2-low patients, while most proliferation-related genes and tyrosine kinase receptor genes are more expressed in HER2-zero tumors. In the HR-positive subgroup, similar gene expression differences were observed. However, in the HR-negative subgroup, no differential gene expression was found between HER2-low and HER2-zero [9]. Zhang et al. also found that 87.5% of the HER2-low subgroup were luminal tumors [41]. The low sensitivity of luminal tumors to chemotherapy may explain the low pCR rate in the HER2-low subgroup. It is interesting that in our study, even in the HR-negative subgroup, the pCR rate of HER2-low patients was lower than that of HER2-zero. Previous studies have found that compared with HER2-zero tumors, HER2-low tumors tend to have lower ki-67 expression and lower histological grade [16, 27, 41]. Dehghani et al. analyzed HER2-low and HER2-zero patients in triple-negative BC and found that HER2-low tumors had lower lymph node involvement rates, less lymphatic invasion, and lower local recurrence rates [42]. So we speculate that even though there is no difference in gene expression between the two groups in the HR-negative subgroup, HER2-low tumors still have low invasiveness, reducing their sensitivity to chemotherapy.
Our analysis shows that HER2-low tumors are better than HER2-zero in both OS and DFS in the overall cohort. Given the role of HER2 in the pathogenesis of breast cancer, we believe that even its expression at low levels would be associated with more aggressive characteristics than its complete absence. However, as mentioned earlier, HER2-low tumors are less aggressive than HER2-zero tumors, both in terms of gene expression and pathological features. This may be related to the high expression of hormone receptors in HER2-low tumors. Most studies have proven that there is an interaction between the signaling pathways of HER2 and hormone receptors. ER signaling can down-regulate HER2 expression, which has a significant impact on HER2-low expression and related tumor biology [43, 44]. The low invasiveness of HER2-low tumors may be the reason for their better prognosis. However, in subgroup analysis, HER2-low had an advantage over HER2-zero in DFS only in the HR-positive group, and no significant differences were noted for OS or DFS in other groups. We speculate that the reason may be related to the low pCR of HER2-low breast cancer. It is possible that the negative impact of low pCR on survival outweighs the positive effect of low tumor aggressiveness on survival in these subgroups.
Traditional anti-HER2 therapies do not benefit HER2-low BC patients [5]. In recent years, HER2-targeted antibody-drug conjugates (ADCs): trastuzumab deruxtecan (T-DXd) and trastuzumab duocarmazine (SYD985) have shown promising anti-tumor activity in patients with HER2-low BC [45, 46]. The results of DESTINY Breast-04 showed that trastuzumab deruxtecan (T-Dxd) significantly improved the objective response rate (52.3% VS. 16.3%) in patients with previously extensively treated HER2-low advanced BC, and prolonged the patients’ PFS and OS [7]. This may be achieved through the so-called “bystander killing” mechanism [47]. Subsequently, the US Food and Drug Administration (FDA) approved T-Dxd as the first targeted therapy for the treatment of patients with unresectable or metastatic HER2-low BC [48]. Since then, the era of binary treatment of HER2 has been broken. DESTINY Break-06 study is another continuous trial for HER2-low metastatic BC designed to evaluate the efficacy and safety of DS-8201 combined chemotherapy in the treatment of HR-positive, HER2-low metastatic BC with failure endocrine therapy. At present, many new ADC drugs (RC48-ADC, ARX788, etc.), BC vaccines (nelipepimut-S, GP2, etc.) and bispecific antibodies (KN026, ZW25, etc.) are being developed, hoping to bring new hope to patients with HER2-low BC [49,50,51,52].
This meta-analysis had a few limitations. First, heterogeneity was found in the analysis. However, we used a random effect model to overcome this. Second, the HR value extracted from survival curves may be less reliable than those directly given by authors. Finally, all the articles we included are retrospective studies, which may potentially induce bias in our results.
Conclusion
In conclusion, our meta-analysis results show that compared with HER2-zero BC, HER2-low BC has a poor response to NACT and a better prognosis in the overall cohort and HR-positive group after NACT. This reminds us that HER-2 low breast cancer has special biological characteristics and requires individualized treatment strategies.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- NACT:
-
Neoadjuvant chemotherapy
- BC:
-
Breast cancer
- HR-positive:
-
Hormone receptor negative
- HR-negative:
-
Hormone receptor positive
- HR:
-
Hazard ratio
- pCR:
-
Pathological complete response
- OS:
-
Overall survival
- DFS:
-
Disease-free survival
References
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast Cancer: correlation of Relapse and Survival with amplification of the HER-2/Neu Oncogene. New York. 1987;235(4785):177–82.
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde V. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–92.
Valero V, Forbes J, Pegram MD, Pienkowski T, Eiermann W, von Minckwitz G, et al. Multicenter phase III randomized trial comparing docetaxel and trastuzumab with docetaxel, carboplatin, and trastuzumab as first-line chemotherapy for patients with HER2-gene-amplified metastatic breast cancer (BCIRG 007 study): two highly active therapeutic regimens. J Clin Oncol. 2011;29(2):149–56.
Gianni L, Lladó A, Bianchi G, Cortes J, Kellokumpu-Lehtinen P-L, Cameron DA, et al. Open-label, phase II, multicenter, randomized study of the efficacy and safety of two dose levels of Pertuzumab, a human epidermal growth factor receptor 2 dimerization inhibitor, in patients with human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28:1131–7.
Fehrenbacher L, Cecchini RS, Geyer CE Jr, Rastogi P, Costantino JP, Atkins JN, et al. NSABP B-47/NRG oncology phase III randomized trial comparing adjuvant chemotherapy with or without trastuzumab in high-risk invasive breast cancer negative for HER2 by FISH and with IHC 1 + or 2. J Clin Oncol. 2020;38:444–53.
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, et al. Human epidermal growth factor receptor 2 testing in breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol. 2018;36(20):2105–22.
Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, et al. Trastuzumab Deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387:9–20.
Hurvitz SA, Wang LS, Chan D, Phan V, Lomis T, McAndrew NP, et al. TRIO-US B-12 TALENT: phase II neoadjuvant trial evaluating trastuzumab deruxtecan with or without anastrozole for HER2-low, HR + early-stage breast cancer. J Clin Oncol. 2022;40(16suppl):TPS623.
Schettini F, Chic N, Brasó-Maristany F, Paré L, Pascual T, Conte B, et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer. 2021;7(1):1.
Rosso C, Voutsadakis LA, Characteristics. Clinical differences and outcomes of breast Cancer patients with negative or low HER2 expression. Clin Breast Cancer. 2022;22(4):391–7.
Denkert C, Seither F, Schneeweiss A, Link T, Blohmer JU, Just M, et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 2021;22:1151–61.
Tarantino P, Hamilton E, Tolaney SM, Cortes J, Morganti S, Ferraro E, et al. HER2-Low breast Cancer: pathological and clinical Landscape. J Clin Oncol. 2020;38:1951–62.
de Moura Leite L, Cesca MG, Tavares MC, Santana DM, Saldanha EF, Guimarães PT, et al. HER2-low status and response to neoadjuvant chemotherapy in HER2 negative early breast cancer. Breast Cancer Res Treat. 2021;190(1):155–63.
Tan R, Ong WS, Lee KH, Lim AH, Park S, Park YH, et al. HER2 expression, copy number variation and survival outcomes in HER2-low non-metastatic breast cancer: an international multicentre cohort study and TCGA-METABRIC analysis. BMC Med. 2022;20:105.
Xu HC, Han YQ, Wu Y, Wang Y, Li Q, Zhang P, et al. Clinicopathological characteristics and prognosis of HER2-Low early-stage breast Cancer: a single-Institution experience. Front Oncol. 2022;12:906011.
Kang S, Lee SH, Lee HJ, Jeong H, Jeong JH, Kim JE, et al. Pathological complete response, long-term outcomes, and recurrence patterns in HER2-low versus HER2-zero breast cancer after neoadjuvant chemotherapy. Eur J cancer. 2022;11:30–40.
Zhou SL, Liu T, Kuang XY, Zhen TT, Shi HJ, Lin Y, et al. Comparison of clinicopathological characteristics and response to neoadjuvant chemotherapy between HER2-low and HER2-zero breast cancer. Breast. 2023;67:1–7.
Alves FR, Gil L, Vasconcelos de Matos L, Baleiras A, Vasques C, Neves MT, et al. Impact of human epidermal growth factor receptor 2 (HER2) low status in response to Neoadjuvant Chemotherapy in early breast Cancer. Cureus. 2022;14(2):e22330.
Di Cosimo S, La Rocca E, Ljevar S, De Santis MC, Bini M, Cappelletti V, et al. Moving HER2-low breast cancer predictive and prognostic data from clinical trials into the real world. Front Mol Biosci. 2022;9:996434.
Shao YB, Yu Y, Luo ZF, Guan HJ, Zhu FY, He YN, et al. Clinical, Pathological Complete Response, and prognosis characteristics of HER2-Low breast Cancer in the Neoadjuvant Chemotherapy setting: a retrospective analysis. Ann Surg Oncol. 2022;29(13):8026–34.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J, et al. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg. 2003;73:712–6.
Armitage P, Berry G, Matthews J. Analysing means and proportions. Statistical methods in Medical Research. Oxford: Blackwell Science; 2002. pp. 83–146.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088e101.
Domergue C, Martin E, Lemarié C, Jézéquel P, Frenel JS, Augereau P, et al. Impact of HER2 status on pathological response after Neoadjuvant Chemotherapy in Early Triple-negative breast Cancer. Cancers (Basel). 2022;14(10):2509.
Qiao WQ, Guo WY, Liu QP, Guo X, Deng XM. Pathological complete response and prognosis after neoadjuvant chemotherapy in patients with HER2-low breast cancer. Ann Diagn Pathol. 2023;64:152125.
Li YJ, Maimaitiaili A, Qu FJ, Li GF, Shi BH, Wang YD, et al. Effect of HER2-low-positive status on neoadjuvant chemotherapy and survival outcome of breast cancer: a 10-year dual-center retrospective study. Am J Cancer Res. 2023;13(8):3571–81.
Pöschke P, Fasching PA, Adler W, Rübner M, Beckmann MW, Hack CC, et al. Clinical characteristics and prognosis of HER2-0 and HER2-Low-positive breast Cancer patients: real-World Data from patients treated with Neoadjuvant Chemotherapy. Cancers (Basel). 2023;15(19):4678.
Li JJ, Yu Y, Ge J. HER2-low-positive and response to NACT and prognosis in HER2-negative non-metastatic BC. Breast Cancer. 2023;30(3):364–78.
Zhong GS, Song DJ, Lou WY, Wei BJ, Chen YM, Cui HD, et al. Pathological complete response rate and clinical outcome after neoadjuvant therapy of HER2-low breast cancer: a National Cancer Database Analysis. Eur J Surg Oncol. 2023;49(11):106970.
Zhang SC, Liu Y, Liu X, Liu YX, Zhang J. Prognoses of patients with hormone receptor-positive and human epidermal growth factor receptor 2-Negative breast Cancer receiving neoadjuvant chemotherapy before surgery: a retrospective analysis. Cancers (Basel). 2023;15(4):1157.
Eiger D, Agostinetto E, Saúde-Conde R, de Azambuja E. The exciting new field of HER2-low breast cancer treatment. Cancers (Basel). 2021;13(5):1015.
Agostinetto E, Rediti M, Fimereli D, Debien V, Piccart M, Aftimos P, et al. HER2-low breast cancer: molecular characteristics and prognosis. Cancers (Basel). 2021;13(11):2824.
Yi XL, Hu SS, Ma ML, Huang DS, Zhang Y. Effect of HER2-low expression on neoadjuvant efficacy in operable breast cancer. Clin Transl Oncol. 2023;13.
Wolff AC, Somerfield MR, Dowsett M, Hammond MEH, Hayes DF, McShane LM, et al. Human epidermal growth factor receptor 2 testing in breast Cancer: ASCO-College of American Pathologists Guideline Update. J Clin Oncol. 2023;41(22):3867–72.
Liu XX, Zhu YF, Li CX, Fang YY, Chen JN, Xu F, et al. Single-cell HER2 quantification via instant signal amplification in microdroplets. Anal Chim Acta. 2023;22:1251.
Baehner FL, Achacoso N, Maddala T, Shak S, Quesenberry CP, Goldstein LC, et al. Human epidermal growth factor receptor 2 assessment in a case-control study: comparison of fluorescence in situ hybridization and quantitative reverse transcription polymerase chain reaction performed by central laboratories. J Clin Oncol. 2010;28(28):4300–6.
Viale G, Slaets L, Bogaerts J, Rutgers E, Van’t veer L, Piccart-gebhart MJ, et al. High concordance of protein (by IHC), gene (by FISH; HER2 only), and microarray readout (by TargetPrint) of ER, PgR, and HER2: results from the EORTC 10041/BIG 03–04 MINDACT trial. Ann Oncol. 2014;25(4):816–23.
Garberis I, Andre F, Magali LT. L’intelligence artificielle pourrait-elle intervenir dans l’aide Au diagnostic des cancers du sein ? - L’exemple De HER2: could artificial intelligence play a role in breast cancer diagnosis? -The example of HER2. Bull Cancer. 2021;108(11S):S1135–45.
Yue M, Zhang J, Wang XR, Yan KZ, Cai LJ, Tian K, et al. Can AI-assisted microscope facilitate breast HER2 interpretation? A multi-institutional ring study. Virchows Arch. 2021;479(3):443–9.
Zhang GC, Ren CY, Li CF, Wang YL, Chen B, Wen LZ, et al. Distinct clinical and somatic mutational features of breast tumors with high-, low-, or non-expressing human epidermal growth factor receptor 2 status. BMC Med. 2022;20:142.
Dehghani M, Keshavarz P, Talei A, Akrami M, Tahmasebi S, Safaie A, et al. The effects of low HER2/neu expression on the clinicopathological characteristics of triple-negative breast Cancer patients. Asian Pac J Cancer Prev. 2020;21(10):3027–32.
Lee AV, Cui X, Oesterreich S. Cross-talk among estrogen receptor, epidermal growth factor, and insulin-like growth factor signaling in breast cancer. Clin Cancer Res. 2001;7:4429s-4435s. discussion 4411s-4412s.
Newman SP, Bates NP, Vernimmen D, Parker MG, Hurst HC. Cofactor competition between the ligand-bound oestrogen receptor and an intron 1 enhancer leads to oestrogen repression of erbB2 expression in breast cancer. Oncogene. 2000;19:490–7.
Nakada T, Sugihara K, Jikoh T, Abe Y, Agatsuma T. The latest research and development into the antibody-drug Conjugate, [Fam-] Trastuzumab Deruxtecan (DS-8201a), for HER2 Cancer therapy. Chem Pharm Bull (Tokyo). 2019;67:173–85.
Banerji U, van Herpen CML, Saura C, Thistlethwaite F, Lord S, Moreno V, et al. Trastuzumab Duocarmazine in locally advanced and metastatic solid tumours and HER2-Expressing breast Cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019;20:1124–35.
Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016;107:1039–46.
FDA approves fam. -trastuzumab deruxtecan-nxki for HER2-low breast cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-fam-trastuzumab-deruxtecan-nxki-her2-low-breast-cancer (accessed on 21 October 2022).
Cardoso F, Dirix L, Conte PF, Semiglazov V, Placido SD, Jaeger M, et al. Phase II study of single agent trifunctional antibody ertumaxomab (anti-HER2 anti-CD3) in HER2 low expressing hormone-refractory advanced breast cancer patients (ABC). Cancer Res. 2010;70(24suppl):3–14.
Mittendorf EA, LU B, Melisko M, Hiller JP, Bondarenko I, Brunt AM, et al. Efficacy and safety analysis of nelipepimut-S vaccine to prevent breast cancer recurrence: a randomized, multicenter, phase III clinical trial. Clin Cancer Res. 2019;25(14):4248–54.
Mittendorf EA, Ardavanis A, Litton JK, Shumway NM, Hale DF, Murray JL, et al. Primary analysis of a prospective, randomized, single-blinded phase II trial evaluating the HER2 peptide GP2 vaccine in breast cancer patients to prevent recurrence. Oncotarget. 2016;7(40):66192–201.
ARX788 in. breast cancer with low expression of HER2-Full text view-ClinicalTrials.gov. https://classic.clinicaltrials.gov/ct2/show/NCT05018676. Accessed July 15, 2023.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Protocol/project development: L.-Y.X and X.-C.C. Data acquisition and interpretation of data: L.-Y.X and Y.Y. Statistics analysis of data: L.-Y.X and Y.Y. Manuscript drafting: L.-Y.X and X.-C.C. Manuscript Revision and accountable for all aspects of the study: L.-Y.X and Y.Y. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xia, LY., Cao, XC. & Yu, Y. Survival outcomes in HER2-low versus HER2-zero breast cancer after neoadjuvant chemotherapy: a meta-analysis. World J Surg Onc 22, 106 (2024). https://doi.org/10.1186/s12957-024-03382-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-024-03382-w