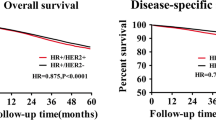
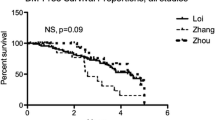
Abstract
Background
The anti-estrogen tamoxifen is a highly effective hormonal therapy for hormonal-positive (HR+) breast cancer patients; however, the estrogen receptor-negative, progesterone receptor-positive (ER–/PR+) subtype does not give the benefits of tamoxifen. Therefore ER–/PR+ breast cancer has a poor clinical outcome, and novel drug therapy for ER–/PR+ breast cancer could benefit these patients.
Methods
53,805 gene expressions were characterized into HR+ BC and triple-negative breast cancer (TNBC) and analyzed through Breast Cancer Gene Expression Miner in 4319 breast cancer patient samples. The clinical outcomes including overall survival, distant metastasis-free survival, and relapse-free survival were obtained from the PrognoScan database containing 1190 human breast cancer patient samples. To determine the function of ERα and inflammation-related genes such as USP1, CDC20, and CASP1, we used the CRISPR–Cas9 system or gene knockdown (KD) system. To check tumor cell proliferation and migration of ERα KO breast cancer cell line, we used tamoxifen and the inflammation inhibitor Ac-YVAD-CHO. For further confirmation, cancer growth was checked with the inflammation inhibitor in ERα KO breast cancer cell line using a three-dimensional (3D) organoid tissue culture system (ex vivo).
Results
We found that gene expression in ER−/PR+ hormonal-positive breast cancer is positively related to ER–/PR– very similar to TNBC, not other HR+ breast cancer using a 4319 breast cancer patient database. Especially, inflammation-related genes, USP1, CDC20, and CASP1, which are highly expressed in TNBC, are also upregulated in ER−/PR+ HR+ breast cancer. Suppression of USP1, CDC20, and CASP1 inhibited tumor cell growth and metastasis in ERα KO (ER–/PR +) cell lines. Interestingly, loss of ERα in HR+ cell lines is not responsive to tamoxifen, but highly sensitive to the inflammation inhibitor, Ac-YVAD-CHO. In in vitro and ex vivo (3D organoid) models, inflammation inhibitor-specific blocks ER–/PR+ tumor proliferation and migration.
Conclusions
These findings suggest that an inflammation inhibitor might be a potential option for therapy for ER–/PR+ HR breast cancer patients.







Similar content being viewed by others
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Onitilo AA, Engel JM, Greenlee RT, Mukesh BN. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res. 2009;7:4–13.
Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–502.
Lim E, MetzgER–Filho O, Winer EP. The natural history of hormone receptor-positive breast cancer. Oncology (Williston Park). 2012;26(688–94):96.
Dai X, Xiang L, Li T, Bai Z. Cancer hallmarks, biomarkers and breast cancer molecular subtypes. J Cancer. 2016;7:1281–94.
El Sayed R, El Jamal L, El Iskandarani S, Kort J, Abdel Salam M, Assi H. Endocrine and targeted therapy for hormone-receptor-positive, HER2-negative advanced breast cancer: insights to sequencing treatment and overcoming resistance based on clinical trials. Front Oncol. 2019;9:510.
Davies C, Godwin J, Gray R, Clarke M, Cutter D, Early Breast Cancer Trialists’ Collaborative G, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–84.
Dai X, Cheng H, Bai Z, Li J. Breast cancer cell line classification and its relevance with breast tumor subtyping. J Cancer. 2017;8:3131–41.
Elledge RM, Green S, Pugh R, Allred DC, Clark GM, Hill J, et al. Estrogen receptor (ER) and progesterone receptor (PgR), by ligand-binding assay compared with ER, PgR and pS2, by immuno-histochemistry in predicting response to tamoxifen in metastatic breast cancer: a Southwest Oncology Group Study. Int J Cancer. 2000;89:111–7.
Hefti MM, Hu R, Knoblauch NW, Collins LC, Haibe-Kains B, Tamimi RM, et al. Estrogen receptor negative/progesterone receptor positive breast cancer is not a reproducible subtype. Breast Cancer Res. 2013;15:R68.
Jordan VC. Tamoxifen: a most unlikely pioneering medicine. Nat Rev Drug Discov. 2003;2:205–13.
Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339:1609–18.
Ravdin PM, Green S, Dorr TM, McGuire WL, Fabian C, Pugh RP, et al. Prognostic significance of progesterone receptor levels in estrogen receptor-positive patients with metastatic breast cancer treated with tamoxifen: results of a prospective Southwest Oncology Group study. J Clin Oncol. 1992;10:1284–91.
Yang LH, Tseng HS, Lin C, Chen LS, Chen ST, Kuo SJ, et al. Survival benefit of tamoxifen in estrogen receptor-negative and progesterone receptor-positive low grade breast cancer patients. J Breast Cancer. 2012;15:288–95.
Bartlett JM, Brookes CL, Robson T, van de Velde CJ, Billingham LJ, Campbell FM, et al. Estrogen receptor and progesterone receptor as predictive biomarkers of response to endocrine therapy: a prospectively powered pathology study in the Tamoxifen and Exemestane Adjuvant Multinational trial. J Clin Oncol. 2011;29:1531.
Beltjens F, Molly D, Bertaut A, Richard C, Desmoulins I, Loustalot C, et al. ER–/PR+ breast cancer: a distinct entity, which is morphologically and molecularly close to triple-negative breast cancer. Int J Cancer. 2021;149:200–13.
Chan M, Chang MC, Gonzalez R, Lategan B, del Barco E, Vera-Badillo F, et al. Outcomes of estrogen receptor negative and progesterone receptor positive breast cancer. PLoS ONE. 2015;10: e0132449.
Ng CH, Pathy NB, Taib NA, Mun KS, Rhodes A, Yip CH. The estrogen receptor negative-progesterone receptor positive breast carcinoma is a biological entity and not a technical artifact. Asian Pac J Cancer Prev. 2012;13:1111–3.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–86.
Dunn JH, Ellis LZ, Fujita M. Inflammasomes as molecular mediators of inflammation and cancer: potential role in melanoma. Cancer Lett. 2012;314:24–33.
Karki R, Man SM, Kanneganti TD. Inflammasomes and Cancer. Cancer. Immunol Res. 2017;5:94–9.
Xia X, Wang X, Cheng Z, Qin W, Lei L, Jiang J, et al. The role of pyroptosis in cancer: pro-cancer or pro-"host"? Cell Death Dis. 2019;10:650.
Liu Z, Li Y, Zhu Y, Li N, Li W, Shang C, et al. Apoptin induces pyroptosis of colorectal cancer cells via the GSDMEdependent pathway. Int J Biol Sci. 2022;18(2):717–730. https://doi.org/10.1007/s12282-023-01437-6
Miguchi M, Hinoi T, Shimomura M, Adachi T, Saito Y, Niitsu H, et al. Gasdermin C is upregulated by inactivation of transforming growth factor β receptor type II in the presence of mutated Apc, promoting colorectal cancer proliferation. PLoS ONE. 2016;11: e0166422.
Gao J, Qiu X, Xi G, Liu H, Zhang F, Lv T, et al. Downregulation of GSDMD attenuates tumor proliferation via the intrinsic mitochondrial apoptotic pathway and inhibition of EGFR/Akt signaling and predicts a good prognosis in nonsmall cell lung cancer. Oncol Rep. 2018;40:1971–84.
Wu M, Wang Y, Yang D, Gong Y, Rao F, Liu R, et al. A PLK1 kinase inhibitor enhances the chemosensitivity of cisplatin by inducing pyroptosis in oesophageal squamous cell carcinoma. EBioMedicine. 2019;41:244–55.
Song C, Kendi AT, Lowe VJ, Lee S. The A20/TNFAIP3-CDC20-CASP1 axis promotes inflammation-mediated metastatic disease in triple-negative breast cancer. Anticancer Res. 2022;42:681–95.
Wei Q, Mu K, Li T, Zhang Y, Yang Z, Jia X, et al. Deregulation of the NLRP3 inflammasome in hepatic parenchymal cells during liver cancer progression. Lab Invest. 2014;94:52–62.
Ma X, Guo P, Qiu Y, Mu K, Zhu L, Zhao W, et al. Loss of AIM2 expression promotes hepatocarcinoma progression through activation of mTOR-S6K1 pathway. Oncotarget. 2016;7:36185–97.
Saeki N, Usui T, Aoyagi K, Kim DH, Sato M, Mabuchi T, et al. Distinctive expression and function of four GSDM family genes (GSDMA-D) in normal and malignant upper gastrointestinal epithelium. Genes Chromosomes Cancer. 2009;48:261–71.
Wang Y, Yin B, Li D, Wang G, Han X, Sun X. GSDME mediates caspase-3-dependent pyroptosis in gastric cancer. Biochem Biophys Res Commun. 2018;495:1418–25.
Simian M, Bissell MJ. Organoids: a historical perspective of thinking in three dimensions. J Cell Biol. 2017;216:31–40.
Fatehullah A, Tan SH, Barker N. Organoids as an in vitro model of human development and disease. Nat Cell Biol. 2016;18:246–54.
Weaver VM, Lelievre S, Lakins JN, Chrenek MA, Jones JC, Giancotti F, et al. beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2:205–16.
Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, Briand P, et al. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci USA. 1998;95:14821–6.
Dowden H, Munro J. Trends in clinical success rates and therapeutic focus. Nat Rev Drug Discov. 2019;18:495–6.
Skardal A, Shupe T, Atala A. Organoid-on-a-chip and body-on-a-chip systems for drug screening and disease modeling. Drug Discov Today. 2016;21:1399–411.
Liu C, Qin T, Huang Y, Li Y, Chen G, Sun C. Drug screening model meets cancer organoid technology. Transl Oncol. 2020;13: 100840.
Gamrani S, Boukansa S, Benbrahim Z, Mellas N, Fdili Alaoui F, Melhouf MA, et al. The Prognosis and Predictive Value of Estrogen Negative/Progesterone Positive (ER–/PR+) phenotype: experience of 1159 primary breast cancer from a single institute. Breast J. 2022;9238804.
Wu N, Fu F, Chen L, Lin Y, Yang P, Wang C. Single hormone receptor-positive breast cancer patients experienced poor survival outcomes: a systematic review and meta-analysis. Clin Transl Oncol. 2020;22:474–85.
Kim JJ, Lee SB, Jang J, Yi SY, Kim SH, Han SA, et al. WSB1 promotes tumor metastasis by inducing pVHL degradation. Genes Dev. 2015;29:2244–57.
Song C, Lowe VJ, Lee S. Inhibition of Cdc20 suppresses the metastasis in triple negative breast cancer (TNBC). Breast Cancer. 2021;28:1073–86.
Song H, Zhao C, Yu Z, Li Q, Yan R, Qin Y, et al. UAF1 deubiquitinase complexes facilitate NLRP3 inflammasome activation by promoting NLRP3 expression. Nat Commun. 2020;11:6042.
Garcia-Santisteban I, Peters GJ, Giovannetti E, Rodriguez JA. USP1 deubiquitinase: cellular functions, regulatory mechanisms and emerging potential as target in cancer therapy. Mol Cancer. 2013;12:91.
Mills JN, Rutkovsky AC, Giordano A. Mechanisms of resistance in estrogen receptor positive breast cancer: overcoming resistance to tamoxifen/aromatase inhibitors. Curr Opin Pharmacol. 2018;41:59–65.
Bardou VJ, Arpino G, Elledge RM, Osborne CK, Clark GM. Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol. 2003;21:1973–9.
Xu S, Li X, Liu Y, Xia Y, Chang R, Zhang C. Inflammasome inhibitors: promising therapeutic approaches against cancer. J Hematol Oncol. 2019;12:64.
Cao Y, Gu ZL, Lin F, Han R, Qin ZH. Caspase-1 inhibitor Ac-YVAD-CHO attenuates quinolinic acid-induced increases in p53 and apoptosis in rat striatum. Acta Pharmacol Sin. 2005;26:150–4.
Schlosser S, Gansauge F, Ramadani M, Beger HG, Gansauge S. Inhibition of caspase-1 induces cell death in pancreatic carcinoma cells and potentially modulates expression levels of bcl-2 family proteins. FEBS Lett. 2001;491:104–8.
Sgroi D, Ma X, Ryan P, Wang Z, Younger J, Isakoff S, et al. Discovery of new gene expression predictors for adjuvant tamoxifen outcome for breast cancer patients. J Clin Oncol. 2004;22:9503.
Jeon YS, Kang SH, Bae YK, Lee SJ. The Clinicopathologic characteristics and clinical outcomes of estrogen receptor negative and progesterone receptor positive breast cancer. J Breast Cancer. 2010;13:74–82.
Parise CA, Caggiano V. Breast cancer survival defined by the ER/PR/HER2 subtypes and a surrogate classification according to tumor grade and immunohistochemical biomarkers. J Cancer Epidemiol. 2014;469251.
Bae SY, Kim S, Lee JH, Lee HC, Lee SK, Kil WH, et al. Poor prognosis of single hormone receptor- positive breast cancer: similar outcome as triple-negative breast cancer. BMC Cancer. 2015;15:138.
Acknowledgements
The authors thank the Jack Kent Cooke Foundation for providing support for the experiments and data analysis. We also thank Zach Cohen (Jack Kent Cooke), Aaron Larson (Mayo High School, Rochester, MN, USA), Corinne Ehrfurth (Mayo High School, Rochester, MN, USA), Christin Stegenga (Mayo High School, Rochester, MN, USA), and JungJin Kim (Mayo Clinic, Rochester, MN, USA) for their contribution in this study.
Funding
This study was funded by the Jack Kent Cooke Foundation’s Young Scholars Program.
Author information
Authors and Affiliations
Contributions
SBL, CS, DWJ, and JYS designed and performed most of the experiments, analyzed data, and prepared the manuscript as a lead author. PSK, ATK, and VL contributed to editing and commenting on the paper. VL and SBL supervised the project.
Corresponding authors
Ethics declarations
Conflict of interest
Dr. Lowe is a consultant for AVID Radiopharmaceuticals, Eisai Co. Inc., Bayer Schering Pharma, GE Healthcare, and Merck Research, and receives research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals, and NIH (NIA, NCI).
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Requests for materials should be addressed to S.B.L (lee.seungbaek@mayo.edu).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Song, C., Kendi, A.T., Shim, J.Y. et al. ER–/PR+ breast cancer is controlled more effectively with an inflammatory inhibitor than hormonal inhibitor. Breast Cancer 30, 436–452 (2023). https://doi.org/10.1007/s12282-023-01437-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-023-01437-6