Abstract
Background
The objective of the CHEOPS trial was to assess the benefit of adding aromatase inhibitor (AI) to metronomic chemotherapy, oral vinorelbine, 50 mg, three times a week for pre-treated, HR + /HER2- metastatic breast cancer patients.
Methods
In this multicentric phase II study, patients had to have progressed on AI and one or two lines of chemotherapy. They were randomized between oral vinorelbine (Arm A) and oral vinorelbine with non-steroidal AI (Arm B).
Results
121 patients were included, 61 patients in Arm A and 60 patients in Arm B. The median age was 68 years. 109 patients had visceral metastases. They all had previously received an AI. The study had been prematurely stopped following the third death due to febrile neutropenia. Median PFS trend was found to be different with 2.3 months and 3.7 months in Arm A and Arm B, respectively (HR 0.73, 95%CI 0.50–1.06, p value = 0.0929). No statistical difference was shown in OS and better tumor response. 56 serious adverse events corresponding to 25 patients (21%) were reported (respectively, 12 (20%) versus 13 (22%) for arms A and B) (NS).
Conclusion
The addition of AI to oral vinorelbine over oral vinorelbine alone in aromatase inhibitor-resistant metastatic breast cancer was associated with a non-significant improvement of PFS. Several unexpected serious adverse events were reported. Metronomic oral vinorelbine schedule, at 50 mg three times a week, requires close biological monitoring. The question of hormonal treatment and chemotherapy combination remains open.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Through their mitogenic effects via the estrogen-receptor alpha (ERα), estrogens play a fundamental role in the carcinogenesis process. Blocking estrogenic signaling is therefore the basic principle of hormone therapy for the treatment of hormone receptor-positive (HR +) breast cancer. Despite the undeniable efficacy of molecules used in endocrine therapy, many tumors have intrinsic or acquired resistance, despite their positive tumor status for ERα. It is nevertheless interesting to note that some clinical studies have shown that 43% and 30% of patients who have relapsed, respectively, on tamoxifen or aromatase inhibitor will respond to fulvestrant treatment, indicating the interest of continuing endocrine therapy beyond neoplastic progression [1, 2].
Systemic chemotherapy with cytotoxic agents remains a standard treatment for metastatic cancer. "Metronomic" chemotherapy is a repeated reduced-dose chemotherapy treatment administered daily for antineoplastic purposes. These effects involve anti-angiogenic impact, interference with immune response and conventional cytotoxic activity [3]. The impact of metronomic chemotherapy on angiogenesis would be explained by the inhibition of the mobilization of endothelial cell progenitors and/or the activation of apoptosis by endothelial cells [4]. There may also be a reactivation of the immune system through a reduction in the number of regulatory T cells, a decrease in their inhibitory function of T and NK lymphocyte activity and a maturation of dendritic cells, thus stimulating the antitumor immune response [5].
Metronomic chemotherapy was initially evaluated in 64 metastatic breast cancer patients by an Italian Phase II study. The treatment included methotrexate and cyclophosphamide and interesting results were reported, with 20% response rate, a 30% clinical benefit rate and no significant toxicity [6]. Regarding metronomic administration of vinorelbine, several regimens with multiple dosages were tested in metastatic breast cancer treatment. Oral vinorelbine according to a 50 mg metronomic regimen, three times a week continuously, was evaluated as monotherapy in a phase I study with pharmacokinetic data [7] and in combination with bevacizumab [8]. These studies showed that administration of 50 mg of oral vinorelbine three times a week was feasible and well tolerated with an interesting clinical benefit in advanced refractory cancers.
The combination of endocrine therapy and metronomic chemotherapy with vinorelbine could therefore be of interest for HR + /HER2− (Hormone receptor positive, human epidermal growth factor receptor 2 negative) breast cancers in a metastatic hormone-resistance setting.
We hypothesized that maintaining HR-targeted therapy after progression in combination with chemotherapy may improve disease control. The CHEOPS study aims to confirm the clinical benefit of a combination of an anti-aromatase and metronomic chemotherapy treatment, oral vinorelbine (OV), 50 mg, three times per week for AI pre-treated, HR + /HER2− metastatic breast cancer patients. It would have the theoretical advantage of being well tolerated and more effective than chemotherapy alone even after an anti-aromatase therapy.
Materials and methods
Population
In this national, multicentric, randomized, open-label phase II study, patients had to have progressed on endocrine therapy and one or two lines of chemotherapy for HR + /HER2− metastatic breast cancer. Inclusion criteria were: age ≥ 50 years, post-menopausal woman, ECOG performance status (PS) 0, 1 or 2, adequate biological function (polynuclear neutrophils ≥ 1,5.109/L; platelets ≥ 100.109/L; creatinine clearance ≥ 30 mL/min; total bilirubin ≤ 1.5 times the upper limit of normal (×ULN); alkaline phosphatases ≤ 2.5 ×ULN; ALAT, ASAT ≤ 1.5 ×ULN in the absence of liver metastases or ≤ 3 ×ULN in the presence of liver metastases), histologically proven breast cancer, progesterone and /or estrogen receptors positive, HER2 negative on primary tumor, patient taking hormonotherapy, in progression, already treated by at least one line of anti-aromatase non-steroidal therapy and by at least one line of chemotherapy and no accessibility to surgical treatment; patient having to begin a second or third line of chemotherapy, no previous treatment containing vinorelbine, presence of one or several measurable(s) or assessable(s) metastatic lesion(s) according to RECIST 1.1; patient with a life expectancy greater than 3 months, without non-irradiated cerebral or symptomatic metastasis, without symptomatic pulmonary carcinomatosis lymphangitis, without known allergies to anastrozole, letrozole or vinorelbine; patient with informed consent signed before enrollment and affiliation to a social security scheme.
Ethics
This study was approved by an ethics committee (Independent Protection Committee 15/026, No. EudraCT 2015–000,401-39) and registered on Clinicantrials.gov as NCT02585388. Patients gave informed consent at the first consultation. The Independent Data Monitoring Committee (IDMC) analyzed interim efficacy data and study-related adverse events.
Study design
Patients were randomized between oral vinorelbine metronomic three times a week (Mondays, Wednesdays, Fridays or Thursdays, Tuesdays, Saturdays) at 50 mg per day in combination with non-steroidal aromatase inhibitor, letrozole 2,5 mg every day or anastrozole 1 mg every day (Arm B) and oral vinorelbine alone (Arm A). Treatments were taken orally until progression of disease or toxicity. Dose adjustment of oral vinorelbine was possible in case of toxicity. Primary outcome measure was progression-free survival (PFS) evaluated every 8 weeks. Secondary outcome measures were evaluation of partial and complete response rate by RECIST 1.1, duration of response, clinical benefit after 24 weeks of treatment, overall survival, toxicity according to criteria NCI CTAEv4.03 evaluated every 4 weeks and health-related quality of life evaluated every 8 weeks with EORTC QLQ-C30 questionnaire.
End points
Progression-free survival (PFS) was defined as time from inclusion to first documentation of objective disease progression or death due to any cause or until the date of the last news (censored data). Evaluation of partial and complete response rate was performed by RECIST 1.1 in each arm. Duration of response was defined as the time from first met for complete or partial response (CR/PR) (whichever is first recorded) until the first date that recurrent or progressive disease which was objectively documented and calculated only in patients with a response to treatment (CR/PR). Clinical benefit is defined by the rate of complete response, the rate of partial response and the stability of lesions at 24 weeks according to criteria RECIST 1.1. Overall survival (OS) was defined as time from inclusion to death due to any cause. Tolerance of the treatment was based on adverse events occurrence according to criteria NCI CTCAEv4.03.
Sample size
To show an increase of median PFS (from 3.5 to 5.5 months, HR 0.636), with unilateral alpha = 5% and power = 80%, 130 evaluable patients were needed for 121 events at the time of the final analysis. Randomization, to a 1:1 ratio, was stratified according to the inclusion center and the number of lines of chemotherapy (second versus third line). All efficacy analyses were conducted on the intending to treat (ITT) population. Two safety interim analyses were scheduled.
Statistics
Qualitative parameters were described in each arm and then compared between the two arms by the Chi2 test or the Fisher exact test depending on the number of patients. Survival parameters (PFS and OS) were estimated using Kaplan–Meier method and described in terms of median associated with two-sided 95% confidence intervals in each arm. Survival distributions were compared between the arms using a log-rank test, supported by a Cox regression. The rates of patients with toxicity, toxicity grade ≥ 3, toxic death, or a serious adverse event will be described by treatment arm and compared according to a Chi2 test or a Fisher test according to the number of patients.
The analyses were performed using SAS software version 9.3 (SAS Institute, Cary, NC).
Results
Population
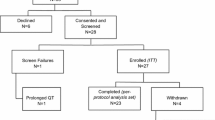
Between October 2015 and May 2017, 27 cancer centers participated in the CHEOPS trial. The participating centers was diverse, with private, public and dedicated cancer centers. Overall, 121 patients were included and randomized: 61 patients received oral vinorelbine (Arm A) and 60 patients a combination of oral vinorelbine and aromatase inhibitor (Arm B) (Fig. 1). The median age was 68 years (range 49–87). The performance status was PS0, PS1 and PS2 for 50 patients (41.7%), 64 patients (53.3%) and 6 patients (5%), respectively. 24 patients (20%) were metastatic at the time of diagnosis. Delay since metastatic diagnosis was 3.2 years (range 0—16.9). 109 patients (90%) had visceral metastases. They all had previously received an aromatase inhibitor; nine patients had received it only in the adjuvant setting. Patients were randomized after one line of chemotherapy (N = 66, 54.5%) or two lines of chemotherapy (N = 55, 45.5%). Seven patients (Arm A: 4; Arm B: 3) had previously received anti-CDK4/6 therapy. Patient features were well balanced between the two treatment arms ( Table 1).
Treatment
The causes of treatment discontinuation are summarized in Fig. 1. The median duration of treatment with vinorelbine was 1.8 months (0.0–15.6) and was similar between the two arms with 1.8 months (0.0–13.3) and 1.9 months (0.3–15.6) in Arm A and B, respectively. Nine patients (7%) had a dose reduction, seven of them for hematological or digestive toxicities. Thirty-five patients (29%) temporarily stopped treatment with vinorelbine, 26 of them due to toxicity. 120 patients (99.2%) definitively discontinued treatment with vinorelbine. For 71 patients (58.7%), the cause of discontinuation of treatment was progression. Other causes of permanent discontinuation of vinorelbine are: patient choice (N = 4), protocol discontinuation (N = 5, patient reached 18 months of post-treatment follow-up), toxicity (N = 18) and other cause (N = 22, mainly at the request of the sponsor following the decision to discontinue the study). Regarding endocrine therapy, 58% of patients were treated with letrozole and 42% with anastrozole.
Primary end point: progression-free survival (PFS)
Median PFS was 2.3 months (95% CI 1.8–3.6) and 3.7 months (95% IC 2.5–4.7) in Arm A and Arm B, respectively (HR 0.73, 95% CI 0.50–1.06, log rank P value = 0.0929) (Fig. 2). The oral vinorelbine–endocrine therapy combination was more effective than oral vinorelbine alone even if statistical significance was not reached.
Secondary end point
Nine patients (5 in Arm A (10%) and 4 in Arm B (8%)) had an objective response to treatment (complete response or partial response). No statistically significant difference was found between the two arms. The median response duration was 3.7 months and 2.8 months in Arm A and Arm B, respectively. No difference was demonstrated between the two arms. Concerning best tumor response, only one complete response was observed in Arm A and none in Arm B. Four patients (7.7%) in each arm obtained a partial response and 19 patients (36.5%) and 30 patients (57.7%) had a stable disease, respectively, in Arm A and Arm B. No statistical difference was found in terms of better tumor response (Fisher exact test, p = 0.122) (Table 2). At 24 weeks, 15 patients (24.6%) and 17 patients (28.3%) were non-progressive in Arm A and Arm B, respectively. Concerning overall survival, with a median follow-up of 16.5 months (2.5–29.4 months), no statistical difference was shown in OS with a median of 17.3 months (95% CI 11.2-NE) and 18.8 months (95% CI 15.0-NE) in Arm A and Arm B, respectively (HR 0.78, 95% CI 0.46–1.33, log-rank P value = 0.3619) (Fig. 3). Among functional scales, comparing the time of diagnosis and at the end of treatment, only three scores were statistically different between the arms in favor of Arm A (oral vinorelbine): physical functioning score (p = 0.034), emotional functioning score (p = 0.017) and social functioning score (p = 0.045). No difference was found for global quality of life score, role functioning score, financial impairment scale and cognitive functioning score. Among symptom scales (fatigue, nausea–vomiting, pain, dyspnea, insomnia, loss of appetite, diarrhea and constipation), only fatigue (p = 0.007), insomnia (0.003) and loss of appetite (p < 0.001) were statistically different in favor of Arm B (oral vinorelbine + AI) (Appendix Table 4).
Main adverse events
Table 3 summarizes all AE grade ≥ 3 reported in at least 10% of patients. The most frequent overall adverse events are: GGT increase (73%), fatigue (67%), high blood pressure (67%), lymphopenia (66%), ASAT increase (59%), anemia (58%) and nausea (53%). At baseline, five patients (8.2%) in Arm A and four patients (6.7%) in Arm B had sensory neuropathy. Only one patient (Arm B) had motor neuropathy. During treatment, sensory neuropathy appeared for two and five additional patients in arms A and B, respectively. 81 patients (67%) had at least one grade of ≥ 3 adverse event (respectively, 40 (66%) versus 41 (68%) for arms A and B). 56 serious adverse events corresponding to 25 patients (21%) were reported (respectively, 12 (20%) versus 13 (22%) for arms A and B): 9 SAE grade 3, 18 SAE grade 4 and 8 SAE grade 5 with 14 severe cytopenia, 9 sepsis, 4 severe digestive disorders, 3 central neurological complications, 2 asthenia, 2 GGT increases and 1 severe pain. Overall occurrence and severe adverse events are detailed in Appendix Table 5. No statistically significant difference was found between the two treatment arms.
The study has been prematurely stopped upon IDMC decision following the third death due to treatment toxicity (febrile neutropenia) after 121 patients were randomized: 1 in Arm A and 2 in Arm B, secondary to febrile neutropenia. Toxic deaths following febrile neutropenia were observed. Patients were 68-year-old, 67-year-old and 80-year-old female patients, each with known diabetes and hypertension. Febrile neutropenia occurred during the first cycle at day 9 and day 10 of vinorelbine + anastrozole for those in Arm B, and at day 30 for the patient in Arm A. Death occurred on day 17 and day 19 in Arm B, and on day 40 in Arm A. Patients experienced: 1) a rapidly unfavorable evolution of sepsis respiratory distress due to E. Coli infection; 2) a refractory septic shock to Pseudomonas aeruginosa complicated with multi-organ failure; 3) craniocerebral injury following pulmonary sepsis, respectively.
Discussion
The results of the CHEOPS study did not reach statistical significance, but showed a modest potential benefit of combining hormone therapy and metronomic chemotherapy in metastatic breast cancer HR + /HER2− pre-treated with endocrine therapy (HR 0.73, 95% CI 0.50–1.06, log-rank P value = 0.0929). Data are still immature due to premature termination due to much higher than expected toxicity.
The limiting factor in this study was the number of toxic deaths induced by oral vinorelbine dose and administration scheme. Indeed, adverse effects of metronomic chemotherapy are most often mild or non-existent and are generally represented by grade 1 toxicities: leukopenia, moderate neutropenia, nausea and vomiting, increased transaminases and asthenia. Serious grade 3–4 toxicities are rare [9, 10]. Several studies have confirmed that metronomic oral vinorelbine can safely be administered at doses up to 50 mg three times a week, especially in advanced breast cancer [11, 12]. Patients with recurrent metastatic breast (BC), prostate (PC) or non-small cell lung cancer (NSCLC) and adequate organ functions were randomly assigned to 30, 40 or 50 mg vinorelbine, taken orally three times a week. With maximum response duration achieved at 50 mg, adverse events were mild and negligible and did not differ between the three arms. Considering the antitumor activity and response duration, the negligible toxicity of the highest dose investigated and the lack of drug accumulation over time, the authors suggest that 50 mg given three times a week is the optimal dose for metronomic oral vinorelbine [11].
The toxic deaths observed here may be the result of poor management of oral oncology drugs at home, for example, by maintaining chemotherapy during periods of neutropenia, infection or hospitalization, despite protocol recommendations. Recommendations for close biological monitoring have been strengthened accordingly (Appendix Table 6). Serious grade 3–4 toxicities would be better managed today through better knowledge of adverse reactions and learning by mistake. The management of per os cancer drugs requires patient autonomy, training of paramedical staff and the knowledge of all health-care providers in contact with the patient. This experience shows that it is now necessary to train non-specialized staff to provide the best possible support to patients treated at home [13,14,15]. The other possibility is to introduce progressively oral vinorelbine with dose escalation scheme or reduce the dose of oral vinorelbine administered three times a week to minimize the risk of toxic death. Oral form of vinorelbine 70 mg/m2 (fractionated on days 1, 3, and 5 for 3 weeks, on and 1 week off, every 4 weeks, for a maximum of 12 cycles) has been experimented in 34 elderly metastatic breast cancer patients and an OR of 38% was reported. Neutropenic infection was evident in two patients (6%). In all instances, these complications resolved during antibiotic therapy [16]. Another study with an alternative on and off metronomic regimen, vinorelbine 30 mg (total dose), one day on and one day off, was given to 32 elderly patients with metastatic breast cancer; a 50% CB was reported, without grade 3 or 4 toxicity [17]. Adamo et al. reported the biological effect of oral metronomic vinorelbine alone or in combination with endocrine therapy in 61 post-menopausal women with untreated stage I–III HR + /HER2-negative breast cancer. Two cases (3.4%) of grade 3 adverse event, both in the oral metronomic vinorelbine alone arm, were observed after completing the 3-week treatment. One case was an acute pancreatitis and the other was an acute gastroenteritis. Overall, no discontinuations due to toxicity was observed. However, the study population was treatment naive, whereas patients in the CHEOPS trial were already at an advanced stage of their metastatic disease (average of 3.2 years since diagnosis of metastatic disease) and had already received one or two lines of chemotherapy [18].
Other combinations may then be possible such as capecitabine and endocrine therapy or oral cyclophosphamide and endocrine therapy. Some studies evaluating combinations were carried out in the 1980s. These randomized trials compared chemotherapy most often with CMF (cyclophosphamide, methotrexate and 5 fluorouracil) as monotherapy with the same chemotherapy in combination with tamoxifen. These different studies showed significant benefit in terms of neoplastic response rate (74% versus 51%, respectively; P < 0.01; 75% versus 49% p = 0.0001) and progression-free survival. However, there was no significant difference in terms of overall survival (111 weeks versus 78 weeks p = 0.25 [19]; 24 months versus 19 months p = 0.07 [20, 21]), mainly due to side effects including thromboembolic effects induced by tamoxifen. However, in patients with metastatic breast cancer, aromatase inhibitor (AI) has been shown to be superior in terms of tamoxifen survival and significantly reduce thromboembolic complications [22]. A randomized phase II clinical trial evaluated the combination of letrozole and cyclophosphamide (50 mg/d for 6 months) compared to letrozole alone in 114 patients with hormone-sensitive metastatic breast cancer. The authors concluded that metronomic cyclophosphamide associated with hormone therapy was beneficial with an objective response rate of 87% in the combination arm versus 71% in the letrozole arm alone [23]. However, these trials mainly excluded patients resistant to concomitant endocrine therapy. In contrast, all patients enrolled in the CHEOPS trial were resistant to AI. This difference may be associated with the small benefit of adding AI to chemotherapy reported here. For HR + /HER2− pre-treated metastatic breast cancer, efficacy of endocrine therapy in combination with chemotherapy remains an open question.
The underlying question is then the interest of maintaining endocrine therapy throughout the treatment, including successive chemotherapy lines. To finish, maintenance endocrine therapy (MET) after chemotherapy could be another way to combine endocrine therapy and chemotherapy. Sutherland et al. discussed four trials addressing the question of whether there is a benefit from introducing endocrine therapy following chemotherapy for metastatic breast cancer [24]. Berrutti et al. investigated the factors influencing response rate and overall survival in 207 MBC patients responding to first-line chemotherapy with epirubicin administration, followed or not by MET. Patients receiving MET survived significantly longer than those submitted to observation in univariate and multivariate analysis [25]. In a phase III randomized trial, Kloke et al. investigated the use of medroxyprogesterone acetate (MPA) maintenance treatment in 90 advanced breast cancer patients with a disease controlled after six cycles of induction chemotherapy. A longer median time to progression (TTP) was reported in the MET arm compared to the observation arm (4.9 months versus 3.0 months), but no difference in OS was observed (17.4 months versus 18.0 months) [26]. Montemmuro et al. retrospectively analyzed the effect of MET after high-dose chemotherapy with hematopoietic progenitor cell transplant (HDCT) on the progression-free survival (PFS) on 109 patients with hormone-dependent MBC who remained progression free for at least 4 months after HDCT. Of these patients, 55 were non-randomly submitted to MET. In multivariate analysis, MET appeared to be a significant factor with improvement of PFS with MET (HR 0.58; 95% CI: 0.362–0.931) [27]. Finally, Dufresne et al. retrospectively identified factors which influence PFS and OS after the first line of chemotherapy in 560 patients with HR + MBC. Administration of MET was shown to improve both PFS (16.3 versus 7.8 months; p < 0.001) and OS (48.1 versus 30 months; p < 0.0001) in multivariate analysis [28]. When chemotherapy for MBC was discontinued due to toxicity, in the absence of progression, the use of ET, with its relatively low toxicity, is a reasonable option, although this approach has not been assessed in randomized trials.
Concerning prior treatment, only a minority of patients had previously received anti-CDK4/6 therapy. This treatment is now the gold standard for first-line treatment of HR + metastatic breast cancer. The results of the CHEOPS trial are therefore not applicable to current daily practice. However, these results do provide a proof of concept on a trend toward an improvement of outcomes with a combination of endocrine therapy and chemotherapy compared to chemotherapy alone. This concept should persist after exposure to anti-CDK4/6.
Conclusions
The addition of aromatase inhibitor to oral vinorelbine over oral vinorelbine alone in aromatase inhibitor resistant metastatic breast cancer was associated with a non-significant improvement of PFS. The study has been prematurely stopped due to treatment toxicity. Several unexpected SAEs were reported. Metronomic oral vinorelbine schedule, at 50 mg three times a week, requires close biological monitoring. The question of hormonal treatment and chemotherapy combination remains open.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- 95% CI:
-
95% Confidence interval
- AE:
-
Adverse event
- AI:
-
Aromatase inhibitor
- CB:
-
Clinical benefit
- CHEOPS:
-
Name of the study
- CR:
-
Complete response
- ERα:
-
Estrogen-receptor alpha
- GINECO:
-
Groupe d'Investigateurs National des Etudes des Cancers Ovariens et du sein (National Investigators Group for Ovarian and Breast Cancer Studies)
- HER2−:
-
Human epidermal growth factor receptor 2 negative
- HR:
-
Hazard ratio
- HR + :
-
Hormone receptor positive
- IDMC:
-
Independent Data Monitoring Committee
- ITT:
-
Intending to treat
- MET:
-
Maintenance endocrine therapy
- NCI CTCAE:
-
Common Terminology Criteria for Adverse Events (CTCAE) elaborated by National Cancer Institute (NCI)
- OR:
-
Objective response
- OS:
-
Overall survival
- OV:
-
Oral vinorelbine
- PFS:
-
Progression-free survival
- PR:
-
Partial response
- PS:
-
Performance status
- RECIST:
-
Response Evaluation Criteria In Solid Tumors
- SAE:
-
Serious adverse event
- SBR Grade:
-
Scarff–Bloom–Richardson grade
- TTP:
-
Time to progression
References
Osborne CK, Pippen J, Jones SE, Parker LM, Ellis M, Come S, et al. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol. 2002;20:3386–95.
Chia S, Gradishar W, Mauriac L, Bines J, Amant F, Federico M, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26:1664–70.
Pasquier E, Kavallaris M, André N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol. 2010;7:455–65.
Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–36.
Koumarianou A, Christodoulou MI, Patapis P, Papadopoulos I, Liakata E, Giagini A, et al. The effect of metronomic versus standard chemotherapy on the regulatory to effector T-cell equilibrium in cancer patients. Exp Hematol Oncol. 2014;3:3.
Colleoni M, Rocca A, Sandri MT, Zorzino L, Masci G, Nolè F, et al. Low-dose oral methotrexate and cyclophosphamide in metastatic breast cancer: antitumor activity and correlation with vascular endothelial growth factor levels. Ann Oncol. 2002;13:73–80.
Briasoulis E, Pappas P, Puozzo C, Tolis C, Fountzilas G, Dafni U. Dose-ranging study of metronomic oral vinorelbine in patients with advanced refractory cancer. Clin Cancer Res. 2009;15:6454–61.
Saloustros E, Kalbakis K, Vardakis N, Kalykaki A, Milaki G, Rovithi M, et al. Metronomic vinorelbine plus bevacizumab as salvage therapy for patients with metastatic breast cancer. J BUON. 2011;16:215–8.
Orlando L, Cardillo A, Rocca A, Balduzzi A, Ghisini R, Peruzzotti G, et al. Prolonged clinical benefit with metronomic chemotherapy in patients with metastatic breast cancer. Anticancer Drugs. 2006;17:961–7.
Lien K, Georgsdottir S, Sivanathan L, Chan K, Emmenegger U. Low-dose metronomic chemotherapy: a systematic literature analysis. Eur J Cancer. 2013;49:3387–95.
Briasoulis E, Aravantinos G, Kouvatseas G, Pappas P, Biziota E, Sainis I, et al. Dose selection trial of metronomic oral vinorelbine monotherapy in patients with metastatic cancer: a hellenic cooperative oncology group clinical translational study. BMC Cancer. 2013;13:263.
Rajdev L, Negassa A, Dai Q, Goldberg G, Miller K, Sparano JA. Phase I trial of metronomic oral vinorelbine in patients with advanced cancer. Cancer Chemother Pharmacol. 2011;68:1119–24.
Bordonaro S, Romano F, Lanteri E, Cappuccio F, Indorato R, Butera A, et al. Effect of a structured, active, home-based cancer-treatment program for the management of patients on oral chemotherapy. Patient Prefer Adherence. 2014;25(8):917–23.
Bonneterre J, Chevalier B, Focan C, Mauriac L, Piccart M. Phase I and pharmacokinetic study of weekly oral therapy with vinorelbine in patients with advanced breast cancer (ABC). Ann Oncol. 2001;12:1683–91.
Depierre A, Freyer G, Jassem J, Orfeuvre H, Ramlau R, Lemarie E, et al. Oral vinorelbine: feasibility and safety profile. Ann Oncol. 2001;12:1677–81.
Addeo R, Sgambato A, Cennamo G, Montella L, Faiola V, Abbruzzese A, et al. Low-dose metronomic oral administration of vinorelbine in the first-line treatment of elderly patients with metastatic breast cancer. Clin Breast Cancer. 2010;10:301–6.
De Iuliis F, Salerno G, Taglieri L, Lanza R, Scarpa S. On and off metronomic oral vinorelbine in elderly women with advanced breast cancer. Tumori. 2015;101:30–5.
Adamo B, Bellet M, Paré L, Pascual T, Vidal M, Pérez Fidalgo JA, et al. Oral metronomic vinorelbine combined with endocrine therapy in hormone receptor-positive HER2-negative breast cancer: SOLTI-1501 VENTANA window of opportunity trial. Breast Cancer Res. 2019;21:108.
Cocconi G, De Lisi V, Boni C, Mori P, Malacarne P, Amadori D, et al. Chemotherapy versus combination of chemotherapy and endocrine therapy in advanced breast cancer. A prospective randomized study Cancer. 1983;51:581–8.
Mouridsen HT, Rose C, Engelsman E, Sylvester R, Rotmensz N. Combined cytotoxic and endocrine therapy in postmenopausal patients with advanced breast cancer. A randomized study of CMF vs CMF plus tamoxifen. Eur J Cancer Clin Oncol 1985; 21: 291–299.
Viladiu P, Alonso MC, Avella A, Beltrán M, Borrás J, Ojeda B, et al. Chemotherapy versus chemotherapy plus hormonotherapy in postmenopausal advanced breast cancer patients. A randomized trial Cancer. 1985;56:2745–50.
Mauri D, Pavlidis N, Polyzos NP, Ioannidis JP. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst. 2006;98:1285–91.
Bottini A, Generali D, Brizzi MP, Fox SB, Bersiga A, Bonardi S, et al. Randomized phase II trial of letrozole and letrozole plus low-dose metronomic oral cyclophosphamide as primary systemic treatment in elderly breast cancer patients. J Clin Oncol. 2006;24:3623–8.
Sutherland S, Miles D, Makris A. Use of maintenance endocrine therapy after chemotherapy in metastatic breast cancer. Eur J Cancer. 2016;69:216–22.
Berruti A, Zola P, Buniva T, Baù MG, Farris A, Sarobba MG et al. Prognostic factors in metastatic breast cancer patients obtaining objective response or disease stabilization after first-line chemotherapy with epirubicin. Evidence for a positive effect of maintenance hormonal therapy on overall survival. Anticancer Res. 1997 ; 17 : 2763–2768.
Kloke O, Klaassen U, Oberhoff C, Hartwich G, Szanto J, Wolf E et al. Maintenance treatment with medroxyprogesterone acetate in patients with advanced breast cancer responding to chemotherapy: results of a randomized trial. Essen Breast Cancer Study Group. Breast Cancer Res. Treat. 1999; 55: 51–59.
Montemurro F, Rondón G, Ueno NT, Munsell M, Gajewski JL, Champlin RE. Factors affecting progression-free survival in hormone-dependent metastatic breast cancer patients receiving high-dose chemotherapy and hematopoietic progenitor cell transplantation: role of maintenance endocrine therapy. Bone Marrow Transpl. 2002;29:861–6.
Dufresne A, Pivot X, Tournigand C, Facchini T, Alweeg T, Chaigneau L, et al. Maintenance hormonal treatment improves progression free survival after a first line chemotherapy in patients with metastatic breast cancer. Int J Med Sci. 2008;5:100–5.
Acknowledgements
We thank the patients, who participated in the trial, and their families. We acknowledge Bénédicte VOTAN, Sébastien ARMANET, Aurélie CHABANON, Sarah SUARD and Amina HAMEK from the GINECO study office. We also thank the following investigators who participated in the trial: Rania BOUSTANY GRENIER, Gaëtan DE RAUGLAUDRE, Yvelise GOUBELY, Julien GRENIER and Alice MEGE (Institut du cancer Avignon-Provence, Avignon), Dominique BERTON, Emmanuelle BOURBOULOUX and Jean-Sébastien FRENEL (Institut de Cancérologie de l’Ouest, Centre René Gauducheau, Saint Herblain), Olivia BALLY, Isabelle RAY-COQUARD, Catherine TERRET and Olivier TREDAN (Centre Léon Bérard, Lyon), Nathalie BONNIN, Claire FALANDRY and Sophie TARTAS (Centre Hospitalier Lyon Sud, Pierre Benite), Stéphane LOPEZ and Oana POP (Centre Hospitalier Annecy Genevois, Pringy), Laurence LANCRY-LECOMTE and Cécile LEYRONNAS (Groupe Hospitalier Mutualiste de Grenoble, Grenoble), Laura DEIANA (Hôpital Morvan, CHU de Brest, Brest), Olfa DERBEL MILED (Hôpital Privé Jean Mermoz, Lyon), Khoutir MAHOUR-BACHA (Hôpitaux du Léman, Thonon-les-Bains), Tifenn L'HARIDON (Centre Hospitalier Départemental Vendée Les Oudairies, La Roche Sur Yon), Dominique SPAETH (ORACLE—Centre d'Oncologie de Gentilly, Gentilly), Oana COJOCARASU (Centre Hospitalier du Mans, Le Mans), and all pharmacists and members of the site study team, as well as Pierre Fabre for their financial support.
Funding
This work was funded by Pierre Fabre.
Author information
Authors and Affiliations
Contributions
Conceptualization and methodology: TB, A-CH-B, P-EH; investigation and resources: all authors except SC; formal analysis: SC; supervision: A-C H-B, P-EH.; writing—original draft: CB and P-EH; writing—review and editing: all authors. Approval of the final version of the manuscript: all authors.
Corresponding authors
Ethics declarations
Conflict of interest
J.-S. F. reports personal fees and non-financial support from Roche, AstraZeneca, Novartis and Pfizer, personal fees from Lilly, GSK and ESAI; T. B. reports grants, personal fees and non-financial support from Novartis, AstraZeneca, and Pfizer, personal fees and non-financial support from Roche, personal fees from SeattleGenetics; C. G. T. reports grants from Roche, personal fees from Astrazeneca, Pfizer and Lilly, non-financial support from MSD and Pfizer; H. S. reports personal fees and non-financial support from Pierre Fabre and Pfizer, personal fees from Novartis and AstraZeneca, non-financial support from Roche; P.-E. H. reports grants from Pfizer, Novartis and Astrazeneca. The other authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Bailleux, C., Arnaud, A., Frenel, JS. et al. CHEOPS trial: a GINECO group randomized phase II assessing addition of a non-steroidal aromatase inhibitor to oral vinorelbine in pre-treated metastatic breast cancer patients. Breast Cancer 30, 315–328 (2023). https://doi.org/10.1007/s12282-022-01426-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-022-01426-1