Abstract
Background
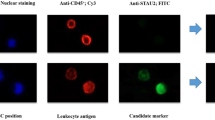
Sensitive and reliable early diagnostic markers for breast cancer (BC) are still unavailable today. In this work, we proposed a new complementary method for detection of BC. This method is based on an observation that lymphocytes re-exposed in vitro to antigenic stimulation express cytoplasmic changes.
Methods
In the new protocol, we recorded changes in the fluorescence intensity of light emitted from lymphocytes obtained from females with and without BC after stimulation with MUC1 antigen utilized flow cytometry.
Results
Out of 55 BC patients tested, 46 were correctly diagnosed. Of 73 controls, 55 were correctly identified as healthy subjects. The sensitivity of the test was 84 %; the specificity was 75 %.
Conclusion
These results suggest a potentially valuable method for detection of BC. The clinical importance of this procedure relies on the ability to screen populations for BC with widely available flow cytometry by a relatively fast, accurate, and economical procedure. Another potential benefit would be identification of candidates for vaccination as a primary or secondary preventive measure.


Similar content being viewed by others
References
Heyderman E, Steele K, Ormerod MG. A new antigen on the epithelial membrane: its immunoperoxidase localisation in normal and neoplastic tissue. J Clin Pathol. 1979;32:35–9.
Singh R, Bandyopadhyay D. MUC1: a target molecule for cancer therapy. Cancer Biol Ther. 2007;6:481–6.
Mukhopadhyay P, Chakraborty S, Ponnusamy MP, Lakshmanan I, Jain M, Batra SK. Mucins in the pathogenesis of breast cancer: implications in diagnosis, prognosis and therapy. Biochim Biophys Acta. 2011;1815:224–40.
Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer. 2009;9:874–85.
Kufe D, Inghirami G, Abe M, Hayes D, Justi-Wheeler H, Schlom J. Differential reactivity of a novel monoclonal antibody (DF3) with human malignant versus benign breast tumors. Hybridoma. 1984;3:223–32.
Levitin F, Stern O, Weiss M, Gil-Henn C, Ziv R, Prokocimer Z, et al. The MUC1 SEA module is a self-cleaving domain. J Biol Chem. 2005;280:33374–86.
Mizejewski GJ. Review of the adenocarcinoma cell surface receptor for human alpha-fetoprotein; proposed identification of a widespread mucin as the tumor cell receptor. Tumour Biol. 2013;34(3):1317–1336.
Hattrup CL, Bradley JM, Kotlarczyk KL, Madsen CS, Hentz JG, Marler RJ, et al. The MUC1 cytoplasmic tail and tandem repeat domains contribute to mammary oncogenesis in FVB mice. Breast Cancer (Auckl). 2008;1:57–63.
Burchell J, Taylor-Papadimitriou J. Effect of modification of carbohydrate side chains on the reactivity of antibodies with core-protein epitopes of the MUC1 gene product. Epithel Cell Biol. 1993;2:155–62.
Keshaviah A, Dellapasqua S, Rotmensz N, Lindtner J, Crivellari D, Collins J, et al. CA15-3 and alkaline phosphatase as predictors for breast cancer recurrence: a combined analysis of seven International Breast Cancer Study Group trials. Ann Oncol. 2007;18:701–8.
Cercek L, Cercek B. Application of the phenomenon of changes in the structuredness of cytoplasmic matrix (SCM) in the diagnosis of malignant disorders: a review. Eur J Cancer. 1977;13:903–15.
Klein O, Linn S, Davidson C, Hadary A, Shukha A, Zidan J, et al. Early detection of malignant process in benign lesions of breast tumor by measurements of changes in structuredness of cytoplasmic matrix in circulating lymphocytes (SCM test) reinduced in vitro by specific tumor antigen. Breast. 2002;11:478–83.
Smorodinsky N, Weiss M, Hartmann ML, Baruch A, Harness E, Yaakobovitz M, et al. Detection of a secreted MUC1/SEC protein by MUC1 isoform specific monoclonal antibodies. Biochem Biophys Res Commun. 1996;228:115–21.
Klein O, Lin S, Embon O, Sazbon A, Zidan J, Kook AI. An approach for high sensitivity detection of prostate cancer by analysis of changes in structuredness of the cytoplasmic matrix of lymphocytes specifically induced by PSA-ACT. J Urol. 1999;161:1994–6.
Nairn RC, Rolland JM. Fluorescent probes to detect lymphocyte activation. Clin Exp Immunol. 1980;39:1–13.
Zurgil N, Deutsch M, Tirosh R, Brodie C. Indication that intracellular fluorescence polarization of T lymphocytes is cell cycle dependent. Cell Struct Funct. 1996;21:271–6.
Maecker HT, Moon J, Bhatia S, Ghanekar SA, Maino VC, Payne JK, et al. Impact of cryopreservation on tetramer, cytokine flow cytometry, and ELISPOT. BMC Immunol. 2005;6:17.
Kurebayashi J, Nishimura R, Tanaka K, Kohno N, Kurosumi M, Moriya T, et al. Significance of serum tumor markers in monitoring advanced breast cancer patients treated with systemic therapy: a prospective study. Breast Cancer. 2004;11(4):389–95.
Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–312.
Agrawal B, Krantz MJ, Reddish MA, Longenecker BM. Cancer-associated MUC1 mucin inhibits human T-cell proliferation, which is reversible by IL-2. Nat Med. 1998;4:43–9.
Deutsch M, Kaufman M, Shapiro H, Zurgil N. Analysis of enzyme kinetics in individual living cells utilizing fluorescence intensity and polarization measurements. Cytometry. 2000;39:36–44.
Afrimzon E, Zurgil N, Shafran Y, Sandbank J, Orda R, Lalchuk S, et al. Monitoring of intracellular enzyme kinetic characteristics of peripheral mononuclear cells in breast cancer patients. Cancer Epidemiol Biomark Prev. 2004;13:235–41.
Rabassa ME, Croce MV, Pereyra A, Segal-Eiras A. MUC1 expression and anti-MUC1 serum immune response in head and neck squamous cell carcinoma (HNSCC): a multivariate analysis. BMC Cancer. 2006;6:253.
Acknowledgements
The authors are very grateful to all participants who donated blood for this research.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Armon-Omer, A., Hadary, A., Hilu, G. et al. Detection of breast cancer from blood through analysis of lymphocyte fluorescent intensity using MUC1 antigen. Breast Cancer 22, 626–633 (2015). https://doi.org/10.1007/s12282-014-0529-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-014-0529-z