Abstract
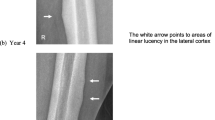
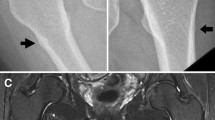
We report here a case of femoral diaphyseal fracture thought to be caused by oversuppression of bone remodeling due to long-term bisphosphonate treatment. The patient was a 63-year-old postmenopausal woman. She had undergone left lumpectomy and sentinel node biopsy for left breast cancer at age 57. The case was diagnosed as pT2N0M0, stage IIA breast cancer. The biopsy sample was positive for hormone receptors and negative for HER2 protein. Postoperatively, exemestane was administered as adjuvant therapy. Right axillary lymph node metastasis was found at age 59, and right axillary lymph node dissection was performed. Postoperatively, epirubicin/cyclophosphamide and paclitaxel were administered. Subsequently, letrozole was administered. However, bone metastases to the first thoracic vertebra and right ilium were found at age 60, and zoledronic acid administration (4 mg/month) for bone metastasis was initiated. The patient developed a transverse fracture in the proximal left femoral diaphysis when she walked on a flat surface after zoledronic acid was administered for 2 years, 10 months. She was treated with an intramedullary nail for left femoral diaphyseal fracture. Cancellous bone of the medullary cavity was histopathologically examined, but there were no metastatic lesions from the breast cancer and no osteoblasts or osteoclasts were observed. Zoledronic acid was immediately discontinued in this patient. In recent years, cases of atypical femoral diaphyseal fractures caused by minor trauma in patients undergoing long-term bisphosphonate treatment have been reported. Thus, careful observation is required for patients who are anticipating bisphosphonate treatment.






Similar content being viewed by others
References
Hortobagyi GN, Theriault RL, Porter L. Efficacy of pamidronate in reducing skeletal complication in patients with breast cancer and lytic bone metastases. Protocol 19 Aredia Breast Cancer Study Group. N Engl J Med. 1996;335:1785–91.
Conte PF, Latreille J, Mauriac L. Delay in progression of bone metastases in breast cancer patients treated with intravenous pamidronate: results from a multinational randomized controlled trial. The Areda Multinational Cooperative Group. J Clin Oncol. 1996;14:2552–9.
Hultborn R, Gundersen S, Ryden S. Efficacy of pamidronate in breast cancer with bone metastases: a randomized, double-blind placebo-controlled multicenter study. Anticancer Res. 1999;19:3383–92.
Theriault RL, Lipton A, Hortobagyi GN. Pamidronate reduces skeletal morbidity in women with advanced breast cancer and lytic bone lesions: a randomized, placebo-controlled trial. Protocol 18 Aredia Breast Cancer Study Group. J Clin Oncol. 1999;17:846–54.
Hortobagyi GN, Theriault RL, Lipton A. Long-term prevention of skeletal complications of metastatic breast cancer with pamidronate. Protocol 19 Aredia Breast Cancer Study Group. J Clin Oncol. 1998;16:2038–44.
Kohno N, Aogi K, Minami H. Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: a randomized, placebo-controlled trial. J Clin Oncol. 2005;23:3314–21.
Odvina CV, Zerwekh JE, Rao DS. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005;90:1294–301.
Kwek EB, Goh SK, Koh JS. An emerging pattern of subtrochanteric stress fractures: a long-term complication of alendronate therapy? Injury. 2008;39:224–31.
Neviaser AS, Lane JM, Lenart BA. Low-energy femoral shaft fractures associated with alendronate use. J Orthop Trauma. 2008;22:346–50.
Odvina CV, Levy S, Rao S. Unusual mid-shaft fractures during long-term bisphosphonate therapy. Clin Endocrinol. 2009;72:161–8.
Cheung RK, Leung KK, Lee KC. Sequential non-traumatic femoral shaft fractures in a patient on long-term alendronate. Hong Kong Med J. 2007;13:484–9.
Ali T, Jay RH. Spontaneous femoral shaft fracture after long-term alendronate. Age Ageing. 2009;38:625–6.
Edwards MH, McCrae FC, Young-Min SA. Alendronate-related femoral diaphysis fracture-what should be done to predict and prevent subsequent fracture of the contralateral side? Osteoporos Int. 2010;21:701–3.
Lenart BA, Lorich DG, Lane JM. Atypical fractures of femoral diaphysis in postmenopausal women taking alendronate. N Engl J Med. 2008;358:1304–6.
Armamento-Villareal R, Napoli N, Diemer K. Bone turnover in bone biopsies of patients with low-energy cortical fractures receiving bisphosphonates: a case series. Calcif Tissue Int. 2009;85:37–44.
Wernecke G, Namburi S, DiCarlo EF. Case report of spontaneous, nonspinal fractures in a multiple myeloma patient on long-term pamidronate and zoledronic acid. HSSJ. 2008;4:123–7.
Russell RG, Rogers MJ. Bisphosphonates: from the laboratory to the clinic and back again. Bone. 1999;25:97–106.
Butler JE, Brown SL, McConnell BG. Subtrochanteric stress fractures in runners. Am J Sports Med. 1982;10:228–32.
Mavrokokki T, Cheng A, Stein B. Nature and frequency of bisphosphonate-associated osteonecrosis of the jaws in Australia. J Oral Maxillofac Surg. 2007;65:415–23.
Komatsubara S, Mori S, Mashiba T. Long-term treatment of incadronate disodium accumulates microdamage but improves the trabecular bone microarchitecture in dog vertebra. J Bone Miner Res. 2003;18:512–20.
Komatsubara S, Mori S, Mashiba T. Suppressed bone turnover by long-term bisphosphonate treatment accumulates microdamage but maintains intrinsic material properties in cortical bone of dog rib. J Bone Miner Res. 2004;19:999–1005.
Dell R, et al. A retrospective analysis of all atypical femur fractures seen in a large Californian HMO from the years 2007 to 2009. ASBMR 2010 Annual Meeting, Toronto.
Black DM, Kelly MP, Genant HK. Bisphosphonates and fractures of the subtrochanteric or diaphyseal femur. N Engl J Med. 2010;362:1761–71.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ishizuna, K., Ota, D., Fukuuchi, A. et al. A case of femoral diaphyseal fracture after long-term treatment with zoledronic acid. Breast Cancer 22, 90–94 (2015). https://doi.org/10.1007/s12282-011-0304-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-011-0304-3