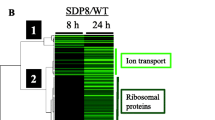
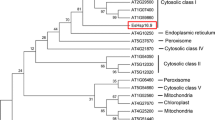
Abstract
Ogataea parapolymorpha (Hansenula polymorpha DL-1) is a thermotolerant methylotrophic yeast with biotechnological applications. Here, O. parapolymorpha genes whose expression is induced in response to heat shock were identified by transcriptome analysis and shown to possess heat shock elements (HSEs) in their promoters. The function of O. parapolymorpha HSF1 encoding a putative heat shock transcription factor 1 (OpHsf1) was characterized in the context of heat stress response. Despite exhibiting low sequence identity (26%) to its Saccharomyces cerevisiae homolog, OpHsf1 harbors conserved domains including a DNA binding domain (DBD), domains involved in trimerization (TRI), transcriptional activation (AR1, AR2), transcriptional repression (CE2), and a C-terminal modulator (CTM) domain. OpHSF1 could complement the temperature sensitive (Ts) phenotype of a S. cerevisiae hsf1 mutant. An O. parapolymorpha strain with an H221R mutation in the DBD domain of OpHsf1 exhibited significantly retarded growth and a Ts phenotype. Intriguingly, the expression of heat-shock-protein-coding genes harboring HSEs was significantly decreased in the H221R mutant strain, even under non-stress conditions, indicating the importance of the DBD for the basal growth of O. parapolymorpha. Notably, even though the deletion of C-terminal domains (ΔCE2, ΔAR2, ΔCTM) of OpHsf1 destroyed complementation of the growth defect of the S. cerevisiae hsf1 strain, the C-terminal domains were shown to be dispensable in O. parapolymorpha. Overexpression of OpHsf1 in S. cerevisiae increased resistance to transient heat shock, supporting the idea that OpHsf1 could be useful in the development of heat-shock-resistant yeast host strains.
Similar content being viewed by others
References
Amin, J., Ananthan, J., and Voellmy, R. 1988. Key features of heat shock regulatory elements. Mol. Cell. Biol. 8, 3761–3769.
Amorós, M. and Estruch, F. 2001. Hsf1p and Msn2/4p cooperate in the expression of Saccharomyces cerevisiae genes HSP26 and HSP104 in a gene- and stress type-dependent manner. Mol. Microbiol. 39, 1523–1532.
Barna, J., Csermely, P., and Vellai, T. 2018. Roles of heat shock factor 1 beyond the heat shock response. Cell. Mol. Life Sci. 75, 2897–2916.
Boy-Marcotte, E., Lagniel, G., Perrot, M., Bussereau, F., Boudsocq, A., Jacquet, M., and Labarre, J. 1999. The heat shock response in yeast: differential regulations and contributions of the Msn2p/Msn4p and Hsf1p regulons. Mol. Microbiol. 33, 274–283.
Chen, J. and Pederson, D.S. 1993. A distal heat-shock element promotes the rapid response to heat-shock of the HSP26 gene in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 268, 7442–7448.
Daggett, V. and Fersht, A. 2003. The present view of the mechanism of protein folding. Nat. Rev. Mol. Cell Biol. 4, 497–502.
Eastmond, D.L. and Nelson, H.C. 2006. Genome-wide analysis reveals new roles for the activation domains of the Saccharomyces cerevisiae heat shock transcription factor (Hsf1) during the transient heat shock response. J. Biol. Chem. 281, 32909–32921.
Fujimoto, M., Takaki, E., Hayashi, T., Kitaura, Y., Tanaka, Y., Inouye, S., and Nakai, A. 2005. Active HSF1 significantly suppresses polyglutamine aggregate formation in cellular and mouse models. J. Biol. Chem. 280, 34908–34916.
Gellissen, G. and Scheffers, L. 2005. Editorial. FEMS Yeast Res. 5, 973–974.
Grably, M.R., Stanhill, A., Tell, O., and Engelberg, D. 2002. HSF and Msn2/4p can exclusively or cooperatively activate the yeast HSP104 gene. Mol. Microbiol. 44, 21–35.
Hahn, J.S., Hu, Z., Thiele, D.J., and Iyer, V.R. 2004. Genome-wide analysis of the biology of stress responses through heat shock transcription factor. Mol. Cell. Biol. 24, 5249–5256.
Harrison, C.J., Bohm, A.A., and Nelson, H.C. 1994. Crystal structure of the DNA binding domain of the heat shock transcription factor. Science 263, 224–227.
Hashikawa, N. and Sakurai, H. 2004. Phosphorylation of the yeast heat shock transcription factor is implicated in gene-specific activation dependent on the architecture of the heat shock element. Mol. Cell. Biol. 24, 3648–3659.
Hill, J., Donald, K.A., and Griffiths, D.E. 1991. DMSO-enhanced whole cell yeast transformation. Nucleic Acids Res. 19, 5791.
Høj, A. and Jakobsen, B.K. 1994. A short element required for turning off heat shock transcription factor: evidence that phosphorylation enhances deactivation. EMBO J. 13, 2617–2624.
Hou, J., Österlund, T., Liu, Z., Petranovic, D., and Nielsen, J. 2013. Heat shock response improves heterologous protein secretion in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 97, 3559–3568.
Inoue, H., Nojima, H., and Okayama, H. 1990. High efficiency transformation of Escherichia coli with plasmids. Gene 96, 23–28.
Jakobsen, B.K. and Pelham, H.R. 1991. A conserved heptapeptide restrains the activity of the yeast heat-shock transcription factor. EMBO J. 10, 369–375.
Kim, H., Thak, E.J., Yeon, J.Y., Sohn, M.J., Choo, J.H., Kim, J.Y., and Kang, H.A. 2018. Functional analysis of Mpk1-mediated cell wall integrity signaling pathway in the thermotolerant methylotrophic yeast Hansenula polymorpha. J. Microbiol. 56, 72–82.
Kim, H., Yoo, S.J., and Kang, H.A. 2015. Yeast synthetic biology for the production of recombinant therapeutic proteins. FEMS Yeast Res. 15, 1–16.
Kurtzman, C.P. 2011. A new methanol assimilating yeast, Ogataea parapolymorpha, the ascosporic state of Candida parapolymorpha. Antonie van Leeuwenhoek 100, 455–462.
Kurylenko, O.O., Ruchala, J., Hryniv, O.B., Abbas, C.A., Dmytruk, K.V., and Sibirny, A.A. 2014. Metabolic engineering and classical selection of the methylotrophic thermotolerant yeast Hansenula polymorpha for improvement of high-temperature xylose alcoholic fermentation. Microb. Cell Fact. 13, 122.
Li, P., Fu, X., Zhang, L., Zhang, Z., Li, J., and Li, S. 2017. The transcription factors Hsf1 and Msn2 of thermotolerant Kluyveromyces marxianus promote cell growth and ethanol fermentation of Saccharomyces cerevisiae at high temperatures. Biotechnol. Biofuels 10, 289.
Manfrão-Netto, J.H.C., Gomes, A.M.V., and Parachin, N.S. 2019. Advances in using Hansenula polymorpha as chassis for recombinant protein production. Front. Bioeng. Biotechnol. 7, 94.
Moon, H.Y., Cheon, S.A., Kim, H., Agaphonov, M.O., Kwon, O., Oh, D.B., Kim, J.Y., and Kang, H.A. 2015. Hansenula polymorpha Hac1p is critical to protein N-glycosylation activity modulation, as revealed by functional and transcriptomic analyses. Appl. Environ. Microbiol. 81, 6982–6993.
Morano, K.A., Grant, C.M., and Moye-Rowley, W.S. 2012. The response to heat shock and oxidative stress in Saccharomyces cerevisiae. Genetics 190, 1157–1195.
Mühlhofer, M., Berchtold, E., Stratil, C.G., Csaba, G., Kunold, E., Bach, N.C., Sieber, S.A., Haslbeck, M., Zimmer, R., and Buchner, J. 2019. The heat shock response in yeast maintains protein homeostasis by chaperoning and replenishing proteins. Cell Rep. 29, 4593–4607.
Mumberg, D., Müller, R., and Funk, M. 1995. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene 156, 119–122.
Nair, R., Shariq, M., Dhamgaye, S., Mukhopadhyay, C.K., Shaikh, S., and Prasad, R. 2017. Non-heat shock responsive roles of HSF1 in Candida albicans are essential under iron deprivation and drug defense. Biochim. Biophys. Acta Mol. Cell Res. 1864, 345–354.
Nicholls, S., MacCallum, D.M., Kaffarnik, F.A.R., Selway, L., Peck, S.C., and Brown, A.J.P. 2011. Activation of the heat shock transcription factor Hsf1 is essential for the full virulence of the fungal pathogen Candida albicans. Fungal Genet. Biol. 48, 297–305.
Nieto-Sotelo, J., Wiederrecht, G., Okuda, A., and Parker, C.S. 1990. The yeast heat shock transcription factor contains a transcriptional activation domain whose activity is repressed under nonshock conditions. Cell 62, 807–817.
Noguchi, C., Watanabe, D., Zhou, Y., Akao, T., and Shimoi, H. 2012. Association of constitutive hyperphosphorylation of Hsf1p with a defective ethanol stress response in Saccharomyces cerevisiae sake yeast strains. Appl. Environ. Microbiol. 78, 385–392.
Pincus, D. 2017. Size doesn’t matter in the heat shock response. Curr. Genet. 63, 175–178.
Ravin, N.V., Eldarov, M.A., Kadnikov, V.V., Beletsky, A.V., Schneider, J., Mardanova, E.S., Smekalova, E.M., Zvereva, M.I., Dontsova, O.A., Mardanov, A.V., et al. 2013. Genome sequence and analysis of methylotrophic yeast Hansenula polymorpha DL1. BMC Genomics 14, 837.
Sakurai, H. and Fukasawa, T. 2001. A novel domain of the yeast heat shock factor that regulates its activation function. Biochem. Biophys. Res. Commun. 285, 696–701.
Scharf, K.D., Berberich, T., Ebersberger, I., and Nover, L. 2012. The plant heat stress transcription factor (Hsf) family: structure, function and evolution. Biochim. Biophys. Acta Gene Regul. Mech. 1819, 104–119.
Schmitt, M.E., Brown, T.A., and Trumpower, B.L. 1990. A rapid and simple method for preparation of RNA from Saccharomyces cerevisiae. Nucleic Acids Res. 18, 3091–3092.
Shi, Y.H., Mosser, D.D., and Morimoto, R.I. 1998. Molecular chaperones as HSF1-specific transcriptional repressors. Genes Dev. 12, 654–666.
Solís, E.J., Pandey, J.P., Zheng, X., Jin, D.X., Gupta, P.B., Airoldi, E.M., Pincus, D., and Denic, V. 2016. Defining the essential function of yeast Hsf1 reveals a compact transcriptional program for maintaining eukaryotic proteostasis. Mol. Cell. 63, 60–71.
Sorger, P.K. 1990. Yeast heat shock factor contains separable transient and sustained response transcriptional activators. Cell 62, 793–805.
Sorger, P.K. and Pelham, H.R.B. 1988. Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell 54, 855–864.
Tamai, K.T., Liu, X., Silar, P., Sosinowski, T., and Thiele, D.J. 1994. Heat shock transcription factor activates yeast metallothionein gene expression in response to heat and glucose starvation via distinct signalling pathways. Mol. Cell. Biol. 14, 8155–8165.
Thak, E.J., Yoo, S.J., Moon, H.Y., and Kang, H.A. 2020. Yeast synthetic biology for designed cell factories producing secretory recombinant proteins. FEMS Yeast Res. 20, foaa009.
Thompson, J.D., Higgins, D.G., and Gibson, T.J. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680.
Treger, J.M., Schmitt, A.P., Simon, J.R., and McEntee, K. 1998. Transcriptional factor mutations reveal regulatory complexities of heat shock and newly identified stress genes in Saccharomyces cerevisiae. J. Biol. Chem. 273, 26875–26879.
Verghese, J., Abrams, J., Wang, Y., and Morano, K.A. 2012. Biology of the heat shock response and protein chaperones: budding yeast (Saccharomyces cerevisiae) as a model system. Microbiol. Mol. Biol. Rev. 76, 115–158.
Vuister, G.W., Kim, S.J., Orosz, A., Marquardt, J., Wu, C., and Bax, A. 1994. Solution structure of the DNA-binding domain of Drosophila heat shock transcription factor. Nat. Struct. Biol. 1, 605–614.
Vydra, N., Toma, A., Glowala-Kosinska, M., Gogler-Piglowska, A., and Widlak, W. 2013. Overexpression of heat shock transcription factor 1 enhances the resistance of melanoma cells to doxorubicin and paclitaxel. BMC Cancer 13, 504.
Wiederrecht, G., Seto, D., and Parker, C.S. 1988. Isolation of the gene encoding the S. cerevisiae heat shock transcription factor. Cell 54, 841–853.
Xu, Y.M., Huang, D.Y., Chiu, J.F., and Lau, A.T.Y. 2012. Post-translational modification of human heat shock factors and their functions: a recent update by proteomic approach. J. Proteome Res. 11, 2625–2634.
Yamamoto, A., Mizukami, Y., and Sakurai, H. 2005. Identification of a novel class of target genes and a novel type of binding sequence of heat shock transcription factor in Saccharomyces cerevisiae. J. Biol. Chem. 280, 11911–11919.
Yamamoto, A. and Sakurai, H. 2006. The DNA-binding domain of yeast Hsf1 regulates both DNA-binding and transcriptional activities. Biochem. Biophys. Res. Commun. 346, 1324–1329.
Yang, D.H., Jung, K.W., Bang, S., Lee, J.W., Song, M.H., Floyd-Averette, A., Festa, R.A., Ianiri, G., Idnurm, A., Thiele, D.J., et al. 2017. Rewiring of signaling networks modulating thermotolerance in the human pathogen Cryptococcus neoformans. Genetics 205, 201–219.
Yeon, J.Y., Yoo, S.J., Takagi, H., and Kang, H.A. 2018. A novel mitochondrial serine O-acetyltransferase, OpSAT1, plays a critical role in sulfur metabolism in the thermotolerant methylotrophic yeast Ogataea parapolymorpha. Sci. Rep. 8, 2377.
Yoo, S.J., Sohn, M.J., Jeong, D.M., and Kang, H.A. 2020. Short bZIP homologue of sulfur regulator Met4 from Ogataea parapolymorpha does not depend on DNA-binding cofactors for activating genes in sulfur starvation. Environ. Microbiol. 22, 310–328.
Acknowledgments
This research was supported by the National Research Foundation of Korea, Grant No. NRF-2017M3C1B5019295 (STEAM Research Project), and Grant No. NRF2018R1A5A1025077 (Advanced Research Center Program). This research was also supported by the Chung-Ang University Graduate Research Scholarship in 2019.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of Interest
We have no conflicts of interest to report.
Supplemental material for this article may be found at http://www.springerlink.com/content/120956.
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Choo, J.H., Lee, SB., Moon, H.Y. et al. Molecular characterization of Hsf1 as a master regulator of heat shock response in the thermotolerant methylotrophic yeast Ogataea parapolymorpha. J Microbiol. 59, 151–163 (2021). https://doi.org/10.1007/s12275-021-0646-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-021-0646-2