Abstract
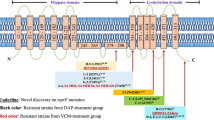
Vancomycin intermediate Staphylococcus aureus (VISA) strains are increasingly prevalent in the hospital setting, and are of major concern in the treatment of methicillin-resistant S. aureus infections. Multiple mutations in vancomycin-susceptible S. aureus (VSSA) strains likely led to the emergence of VISA, and point mutations in the agr, orf1, yvqF, vraSR, graSR, and tcaRAB genes of VISA strains have been shown to contribute to glycopeptide resistance. Therefore, we investigated point mutations in these genes from 87 VISA and 27 VSSA clinical strains isolated from Korean hospitals. All strains were assigned an agr type (I, II, or III) on the basis of multiplex PCR, with the majority of VISA strains belonging to agr groups I and II. Sequencing revealed amino acid changes in vraS from VISA strains which were not present in the VSSA strains. The E59D substitution in the vraR gene occurred in 36.3% of VSSA/agrI and 92.7% of VISA/agrI strains, suggesting that this mutation associated with emergence of VISA/agrI strains. VISA strains were classified into 31 mutation patterns according to mutations in the yvqF, vraSR, graSR, and tcaRAB genes. In addition, the mutation patterns were correlated with agr and sequence type (ST). The most prevalent pattern included agr type I (ST 72) strains with E59D (vraR), L26F and T224I (graS), D148Q (graR), and L218P, R283H and G312D (tcaA) amino acid substitutions. The minimum inhibitory concentration (MIC) range of mutation pattern 5 toward oxacillin and imipenem was much lower than that of patterns 6 and 24. These results improve our understanding of emergence of VISA strains.
Similar content being viewed by others
References
Chung, G.T., Cha, J.O., Han, S.Y., Jang, H.S., Lee, K.M., Yoo, J.I., Yoo, J.S., Kim, H.B., Eun, S.H., Kim, B.S., et al. 2010. Nationwide surveillance study of vancomycin intermediate Staphylococcus aureus strains in Korean hospitals from 2001 to 2006. J. Microbiol. Biotechnol. 20, 637–642.
Clinical and Laboratory Standards Institute (CLSI). 2007. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Approved standard, 7th ed. Clinical and Laboratory Standards Institute Document M100-S20. Clinical and Laboratory Standards Institute, Wayne, PA, USA.
Cui, L., Lian, J.Q., Neoh, H.M., Reyes, E., and Hiramatsu, K. 2005. DNA microarray-based identification of genes associated with glycopeptide resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 49, 3404–3413.
Cui, L., Neoh, H.M., Shoji, M., and Hiramatsu, K. 2009. Contribution of vraSR and graSR point mutation to vancomycin resistance in vancomycin intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 53, 1231–1234.
Deresinski, S. 2005. Methicillin-resistant Staphylococcus aureus: An evolutionary, epidemiologic, and therapeutic odyssey. Clin. Infect. Dis. 40, 562–573.
Enright, M.C., Day, N.P., Davies, C.R., Peacock, S.J., and Spratt, B.G. 2000. Multilocus sequencing typing for characterization of methicillin resistant and methicillin susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 38, 1008–1015.
Gilot, P., Lina, G., Thierry, C., and Poutrel, B. 2002. Analysis of the genetic variability of genes encoding the RNA III-activating components Agr and TRAP in a population of Staphylococcus aureus strains isolated from cows with mastitis. J. Clin. Microbiol. 40, 4060–4067.
Hiramatsu, K., Aritaka, N., Hanaki, H., Shiori, K., Yasuyuki, H., Satoshi, H., Yoshinosuke, J., and Kobayashi, I. 1997. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet 350, 1670–1673.
Hiramatsu, K., Okuma, K., Ma, X.X., Yamamoto, M., Hori, S., and Kapi, M. 2002. New trends in Staphylococcus aureus infections: Glycopeptide resistance in hospital and methicillin resistance in the community. Curr. Opin. Infect. Dis. 15, 407–413.
Howden, B.P., Davies, J.K., Johnson, P.D., Stinear, T.P., and Gravson, M.L. 2010. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin intermediate and heterogeneous vancomycin intermediate strains: Resistance mechanisms, laboratory detection, and clinical implications. Clin. Microbiol. Rev. 23, 99–139.
Jansen, A., Turck, M., Szekat, C., Naqel, M., Clever, I., and Bierbaum, G. 2007. Role of insertion elements and yycFG in the development of decreased susceptibility to vancomycin in Staphylococcus aureus. Int. J. Med. Microbiol. 297, 205–215.
Kato, Y., Suzuki, T., Ida, T., and Maebashi, K. 2010. Genetic changes associated with glycopeptide resistance in Staphylococcus aureus: Predominance of amino acid substitutions in YvqF/VraSR. J. Antimicrob. Chemother. 65, 37–45.
Kuroda, M., Kuroda, H., Oshima, T., Takeuchi, F., Mori, H., and Hiramatsu, K. 2003. Two-component system VraSR positively modulates the regulation of cell-wall biosynthesis pathway in Staphylococcus aureus. Mol. Microbiol. 49, 807–821.
Maki, H., McCallum, N., Bischoff, M., Wada, A., and Brigitte, B.B. 2004. tcaA Inactivation increases glycopeptide resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 48, 1953–1959.
McCallum, N., Brassinga, A.K., Sifri, C.D., and Brigitte, B.B. 2007. Functional characterization of TcaA: Minimal requirement for teicoplanin susceptibility and role in Caenorhabditis elegans virulence. Antimicrob. Agents Chemother. 51, 3836–3843.
Meehl, M., Herbert, S., Gotz, F., and Cheung, A. 2007. Interaction of the GraRS two-component system with the VraFG ABC transporter to support vancomycin-intermediate resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 51, 2679–2689.
Moellering Jr., R.C., 2005. The management of infections due to drug-resistant Gram-positive bacteria. Eur. J. Clin. Microbiol. Infect. Dis. 24, 777–779.
Neoh, H.M., Cui, L., Yuzawa, H., Takeuchi, F., Matsuo, M., and Hiramatsu, K. 2008. Mutated response regulator graR is responsible for phenotypic conversion of Staphylococcus aureus from heterogeneous vancomycin intermediate resistance to vancomycin intermediate resistance. Antimicrob. Agents Chemother. 52, 45–53.
Renzoni, A., Kelley, W.L., Barras, C., Monod, A., Huggler, E., Francois, P., Schrenzel, J., Studer, R., Vaudaux, P., and Lew, D.P. 2009. Identification by genomic and genetic analysis of two new genes playing a key role in intermediate glycopeptide resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 53, 903–911.
Sakoulas, G., Eliopoulos, G.M., Moellering, R.C., Wennerstn, C., Venkataraman, L., Novick, R.P., and Gold, H.S. 2002. Accessory gene regulator (agr) locus in geographically diverse Staphylococcus aureus isolates with reduced susceptibility to vancomycin. Antimicrob. Agents Chemother. 46, 1492–1502.
Seidl, K., Stucki, M., Ruegg, M., Goerke, C., Wolz, C., Harris, L., Berger, B.B., and Bischoff, M. 2006. Staphylococcus aureus CcpA affects virulence determinant production and antibiotic resistance. Antimicrob. Agents Chemother. 50, 1183–1194.
Singh, V.K., Schmidt, J.L., Jayaswal, R.K., and Wilkinson, B.J. 2003. Impact of sigB mutation on Staphylococcus aureus oxacillin and vancomycin resistance varies with parental background and method of assessment. Int. J. Antimicrob. Agents 21, 256–261.
Tenover, F.C. and Moellering, R.C. 2007. The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clin. Infect. Dis. 44, 1208–1215.
Yamakawa, J., Aminaka, M., Okuzumi, K., Kobayashi, H., Katayama, Y., Kondo, S., Oquri, T., Hori, S., Cui, L., Ito, T., et al. 2012. Heterogeneously vancomycin intermediate Staphylococcus aureus (hVISA) emerged before the clinical introduction of vancomycin in Japan: A retrospective study. J. Infect. Chemother. 18, 406–409.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoo, J.I., Kim, J.W., Kang, G.S. et al. Prevalence of amino acid changes in the yvqF, vraSR, graSR, and tcaRAB genes from vancomycin intermediate resistant Staphylococcus aureus . J Microbiol. 51, 160–165 (2013). https://doi.org/10.1007/s12275-013-3088-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-013-3088-7