Abstract
The emergence of vancomycin-resistant Staphylococcus aureus (VRSA) threatens global health. The mechanism of vancomycin resistance of VRSA without vanA gene acquisition was not fully elucidated. Therefore, we aimed to determine the mechanism of vancomycin resistance of VRSA besides that by vanA gene acquisition. In this study, we obtained vancomycin-resistant strains (V036-V64; MIC = 64 µg /ml) from susceptible strain (V036; MIC = 0.5 µg /ml) by exposure of vancomycin in vitro and examined the phenotypic characteristics and antibiotic susceptibility profiles of the resistant strain (V036-V64). To identify the genetic variations caused vancomycin resistance, we determined the complete genome sequences of V036 and V036-V64 and analyzed for single-nucleotide polymorphisms (SNPs) between two strains. Morphologically, V036-V64 had a twofold thicker cell wall compared with V036. Linezolid, rifampicin, and ceftaroline had similar MIC ranges against V036-V64 and V036, but V036-V64 showed lower susceptibilities to daptomycin and telavancin. We detected eight single-nucleotide polymorphisms differing between V036-V64 and V036: rimM (G16D), ssaA2 (G128A), rpsK (P60R), rpoB (R917C), walK (T492R), d-alanyl-d-alanine carboxypeptidase (L307I), vraT (A152V), and chromosome segregation ATPase (T440I). This study demonstrates that, under selective pressure, by the accumulation of mutations in genes related to cell wall synthesis, vancomycin-susceptible S. aureus can develop thicker cell walls and, hence, develop high vancomycin resistance. Thus, we highlight a novel vanA-negative mechanism for VRSA emergence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) is a major cause of nosocomial and community-associated infections throughout the world. MRSA can cause a variety of problems, from skin infections, sepsis, and pneumonia to bloodstream infections. Vancomycin is the main antimicrobial agent available to treat serious infections with MRSA. However, the intensive use of vancomycin over the years in healthcare premises has led to the development of strains with reduced vancomycin susceptibility.
Staphylococcus aureus strains with reduced vancomycin susceptibility are classified into two types depending on the level of vancomycin resistance. The first type is vancomycin-intermediate S. aureus (VISA), which was first reported in Japan in 1997 and against which vancomycin has a moderate minimum inhibitory concentration (MIC) (4–8 μg/ml) (Hiramatsu et al. 1997). VISA isolates do not carry imported foreign genetic elements; rather, the high vancomycin MIC values are related to the accumulation of mutations (Howden et al. 2014). The second type of strain is VRSA, first reported in the United States in 2002; the MIC of vancomycin is extremely high MIC (≥ 16 μg/ml) against VRSA (Sievert et al. 2008). Resistance in VRSA is conferred by the acquisition of the vanA gene by transposon Tn1546 and plasmid transfer from vancomycin-resistant Enterococcus faecalis (Sievert et al. 2008). This vanA-type of VRSA is rare and geographically limited (Sievert et al. 2008; Tenover 2008). However, VISA strains are increasingly reported throughout the world (Howden et al. 2014).
It is noteworthy that S. aureus with reduced susceptibility to vancomycin is not restricted to humans. Recently, VRSA and VISA were reported to have been isolated from animals such as cattle, goats, and pigs (Adegoke et and Okoh 2014; Bhattacharyya et al. 2016; Kwok et al. 2013; Moreno et al. 2016).
In VISA, several gene mutations are known to contribute to the development of vancomycin resistance, including ropB (encoding a DNA-dependent RNA polymerase β-subunit), walKR, vrsSR, and graSR (encoding two-component regulatory systems) (Howden et al. 2010, 2014; Saito et al. 2014; Hu et al. 2016; Alam et al. 2014). However, the mechanisms underlying the progression of vancomycin resistance in VRSA by means other than vanA gene acquisition are not yet clear.
In this study, we aimed to examine the phenotypic characteristics and antibiotic susceptibility profiles of an induced vancomycin-resistant mutant and to identify the genetic variations causing vancomycin resistance.
Materials and methods
In vitro selection of the induced vancomycin-resistant mutant
A clinical isolate of vancomycin-susceptible S. aureus (VSSA) (V036; vancomycin MIC = 0.5) with sequence type (ST) 5 and staphylococcal cassette chromosome mec type II was used as the parental strain. Using V036 as the parental strain, a vancomycin-resistant mutant (V036-V64) was selected in vitro by serial passaging with progressively increasing concentrations of vancomycin. Briefly, overnight cultures of the vancomycin-susceptible strain in tryptic soy broth (TSB) were diluted 1:100 into fresh medium containing sub-inhibitory concentrations of vancomycin increased serially and incubated with shaking at 37 °C. To obtain a single vancomycin-resistant population, mutants grown in TSB containing 32 μg/ml vancomycin were selected again on tryptic soy agar (TSA) plates containing 64 μg/ml vancomycin. The vancomycin MICs of individual colonies picked at random were confirmed by the E test (bioMérieux, Marcy-I’Étoile, France). As a result, a stable mutant with a vancomycin MIC value of 64 μg/ml (V036-V64) was obtained. Chromosomal DNA digests (SmaI) of the strains V036-V64 and V036 showed identical pulsed-field gel electrophoretic profiles (data not shown). The mutant strain grown from a single colony was stored in 20% glycerol at − 80 °C.
Antimicrobial susceptibility testing
Susceptibilities to ampicillin, azithromycin, clindamycin, cefoxitin, ciprofloxacin, daptomycin, erythromycin, gentamicin, linezolid, mupirocin, oxacillin, penicillin, rifampicin, quinupristin-dalfopristin, trimethoprim-sulfamethoxazole, teicoplanin, and vancomycin were tested using microdilution panels (MicroScan, Beckman Coulter Inc., Brea, CA, USA). Ceftaroline and telavancin MIC values were determined using the MIC test strip (Liofilchem, Roseto degli Abruzzi, Italy) and the Oxoid M.I.C.Evaluator™ (M.I.C.E™; Thermo Fisher Scientific, Basingstoke, England), respectively. The plates were incubated at 35 °C and read after 48 h. Population analysis was performed according to a reported method (Saito et al. 2014). Briefly, about 107–108 colony-forming units (CFU) of the overnight culture of each strain were inoculated on TSA plates containing various concentrations of vancomycin. The numbers of colonies formed after 48 h incubation at 35 °C were counted and plotted in a semilogarithmic graph.
DNA extraction
Genomic DNA was extracted by using a Wizard genomic DNA preparation kit (Promega, Madison, WI, USA) according to the manufacturer’s protocol for bacterial cells. Lysostaphin was added at a final concentration of 30 µg/ml in lysis buffer, followed by incubation for 1 h at 37 °C. DNA quality and quantity were evaluated by agarose gel electrophoresis and using a Nanodrop (Thermo Fisher Scientific) spectrophotometer, respectively.
Whole-genome sequencing and annotation
Whole-genome sequencing (WGS) for all strains was performed using an Illumina MiSeq system (Illumina Inc., San Diego, CA, USA). The sequencing library was prepared with the TruSeq DNA LT Sample Prep kit (Illumina) according to the manufacturer’s instructions. Sequencing was performed with 300 bp paired-end reads to a coverage of over 200X. The generated paired-end sequencing reads were assembled using SPAdes 3.10.0 (Bankevich et al. 2012). Gene prediction was performed using Glimmer 3 (Delcher et al. 2002), and annotation was conducted by homology search against the Clusters of Orthologous Groups (COG) and SEED databases (Disz et al. 2010; Tatusov et al. 1997). For validation, the assembled sequences were compared with the reference genomes of two MRSA strains, N315 and Mu50.
Single-nucleotide polymorphism calling
MUMmer version 3.23 (Delcher et al. 2002) was used to align each of the de novo assemblies to a reference genome. Single-nucleotide polymorphisms (SNPs) were then called from the resulting alignments with a MUMmer application named show-snps (with options-Clr).
Nucleotide sequence accession numbers
The complete nucleotide sequences of strains V036 and V036-V64 were submitted to GenBank under accession numbers PJIG00000000 and PJIH00000000, respectively.
Results
Characterization of the V036-V64 strain
The colony size of V036-V64 (1 mm) on the plate was smaller than that of V036 (3 mm). The colonies of V036-V64 on the plate were homogeneous in size, indicating the genetic stability of the strain (Fig. S1). Thickened cell wall peptidoglycan layers are the mechanistic features of the VISA phenotype. To evaluate the change in cell wall thickness in V036-V64, transmission electron microscopy was performed, which revealed that V036-V64 (34.96 ± 7.73 nm) had a thicker cell wall compared to V036 (17.71 ± 1.97 nm) (Fig. S1). Furthermore, the V036-V64 strain grew extremely slowly (Fig. S2). The average ± standard deviation DTs of the V036 and V036-V64 strains were 32.3 ± 3.9 min and 79.1 ± 3.0 min, respectively. Next, the V036-V64 strain had higher vancomycin resistance than that of the parent V036 strain; V036-V64 exhibited about 106 CFU at a vancomycin concentration of 64 μg/ml (Fig. S2). Because the growth rate of the V036-V64 strain was too slow for us to determine the MIC after 24 h, the MICs of both V036 and V036-V64 strains were assessed after 48 h. The MICs of most of the tested antibiotics, including beta-lactams, macrolides, lincosamides, and fluoroquinolones, were unchanged in V036-V64 compared to V036 strain (Table 1). Meanwhile, the MICs of teicoplanin (glycopeptide) and telavancin (lipoglycopeptide) against V036-V64 were 16-fold and 23-fold higher, respectively, than those against V036. Daptomycin (lipopeptide) also had a higher MIC against the V036-V64 strain.
Genetic changes in the VRSA phenotype compared with the VSSA strain V036
We determined the complete nucleotide sequences of the strains V036 and V036-V64 as 2,770,222 bp and 2,770,222 bp, respectively. The 2800 genes (2274 coding sequences, two pseudogenes, 16S rRNAs, and 58 tRNAs) were annotated with the MiGAP program. The GC content of V036 was 32.8%. This gene number and GC percentage were very similar to those of the N315 sequence (2787 genes and GC content of 32.8%).
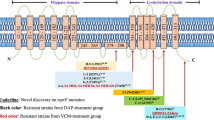
To investigate the genetic changes in V036-V64 compared to V036, we analyzed the SNPs between the two strains. Remarkably, only eight SNPs were detected between V036 and V036-V64 (Table 2). The eight detected SNPs were non-synonymous substitutions, i.e., G16D, G128A, P60R, R917C, T492R, L307I, A152V, and T440I, located in rimM, ssaA2, rpsK, rpoB, walK, pbp4, vraT, and chromosome segregation ATPase genes, respectively.
Discussion
The prevalence of superbugs, such as MRSA and even VRSA, is increasing rapidly both in hospitals and in the community. To date, 12 VRSA isolates have been reported in the United States, all of which belong to a phylogenetic lineage known as clonal complex 5 (Sievert et al. 2008). Although the majority of reports have been limited to the United States, with a few from other parts of the world (e.g., Iran) (Shekarabi et al. 2017; Thati et al. 2011; Tiwari and Sen 2006), the recent detection of a relatively high frequency of VRSA indicates the possibility of the dissemination of VRSA strains in various parts of the world. Furthermore, vanA-negative strains among these VRSA strains have also been reported. However, vancomycin resistance mechanisms of VRSA other than vanA gene acquisition, especially the underlying genetic changes, are still not understood fully.
In this study, to gain enhanced understanding of the genetic characteristics of the vancomycin resistance of VRSA, we obtained a vancomycin-resistant strain with the same genetic background as that of the susceptible strain ST5 by vancomycin selection in vitro. Next, we performed WGS of VSSA and its isogenic mutant VRSA strain and analyzed the genetic variations between the two strains. Herein, we identified eight non-synonymous mutations in V036-V64, including G16D in rimM, G128A in ssaA2, P60R in rpsK, R917C in rpoB, T492R in walK, L307I in SAV036_02325 (encoding d-alanyl-d-alanine carboxypeptidase), A152V in vraT, and T440I in SAV036_02818 (encoding chromosome segregation ATPase). RimM, a ribosomal maturation factor, and the ribosomal protein S2 are important proteins involved in protein synthesis. More specifically, RimM is an accessory factor required for 30S maturation and assembly in Escherichia coli (Lӧvgren et al. 2004), and the deletion of rimM has been known to decrease the growth rate and reduce translational efficiency at 37 °C (Bylund et al. 2001). rpsK encodes the 30S ribosomal protein S11. Mutation of this gene showed cell separation, swimming defects, and biofilm formation acceleration in Bacillus subtilis (Takada et al. 2014). The mutation of these two genes and SAV036_02818 (encoding chromosome segregation ATPase) might affect the growth rate of the V036-V64 strain via decreased chromosome segregation and cell separation in the V036-V64 strain. S. aureus SsaA homologs have been reported to share a common cysteine, histidine-dependent aminohydrolase/peptidase-amidase domain, and amino-terminal signal sequences, indicating that they are likely to be exported and/or targeted to the cell wall or membrane. SsaA is involved in cell wall metabolism and is regulated by the walKR two-component system in S. aureus (Dubrac et al. 2007; Hu et al. 2016). In addition, we found several mutated genes that were previously reported in VISA, such as vraT, walK, and rpoB involved in transcriptional regulation (Bankevich et al. 2012; Boyle-Vavra et al. 2013). VraTSR is a three-component system that regulates cell wall stress stimulon and the VraT is a negative regulator of VraSR (Hu et al. 2016). The VraT-Y220C mutation mediates the vancomycin-resistant phenotype in the ST8-USA300 strain of the VISA isogenic series of SG-S, SG-R, and SG-rev (Gardete et al. 2012). As the only known essential two-component regulatory system for the viability of S. aureus, the WalKR system connects cell wall biosynthesis with cell division (Howden et al. 2011). It has been best studied in B. subtilis, S. aureus, and Streptococcus pneumoniae where cell wall metabolism genes dominate the predicted regulon of walR (Howden et al. 2011). The walKR mutation is the most frequent in VISA strains (Howden et al. 2011). Several mutations within the WalK, including G223D, V268F, N382S, A567D, and T595I, have been reported (Howden et al. 2011), and the positions of mutations are not limited to specific domain or region. In this study, the R492C mutation of walK in V036-V64 strain was detected, which was different from those previously described in VISA. This suggests that the novel mutation sites were important for the development of resistance to vancomycin beyond VISA levels.
Recently, Ishii et al. reported the experimental development of vancomycin-resistant strains (Ishii et al. 2015). They generated a highly vancomycin-resistant S. aureus mutants by exposure with ethyl methanesulfonate. Eleven genes, including graS, ausA, rpoB, rpoC, yloV, ebhA, SA1593, perR, sdrC, terC, and SA1584, were mutated in two high-MIC (vancomycin MIC = 32 μg/ml) strains of ST5. Among them, only one gene (ropB) was consistent with our results. However, the mutation sites in ropB gene were different each other; L1083F in VR3, M978I in VR7 (Ishii et al. 2015), and R917C in our study. Two mutation sites of M978I and R917C were located in the RNA polymerase Rpb2 domain 6 (position 674-1069). This domain contains the important structural motifs, switch 3 and the flap loop, and binds an active site metal. This domain is also involved in binding to Rpb1 and Rpb3 (Cramer et al. 2001). The mutation site of H481Y previously reported in VISA (Alam et al. 2014) is located in domain 3 (position 468-536). This domain is known as the fork domain and is proximal to catalytic sites (Cramer et al. 2001). These suggest that the development pathways for obtaining high vancomycin resistance beyond intermediate resistance could vary. Above all, mutations detected in vancomycin-resistant strains need to be verified experimentally.
Although the genomic information from whole-genome sequencing approach are powerful hypothesis generator, genetic manipulation experiments are necessary to confirm functions of individual genes. In this study, a prospective trial was performed using two-step allelic exchange method described previously (Bae and Schneewind 2006), which requires cloning of the upstream and downstream sequences of a locus of interest into a pKOR1 plasmids. However, eventually failed to obtain knockout mutant strains.
Staphylococcus aureus V036-V64 strain was difficult to work due to two barriers in genetic manipulation experiments. First, a major barrier to the genetic manipulation of Staphyococci is the inability to transform plasmid DNA into the majority of clinical isolates due to a strong restriction-modification (RM) barrier. We have observed that S. aureus V036-V64 strain had type I RM system encoded by a sau1hsdR gene on the chromosome and by two copies of sau1hsdM and sau1hsdS on genomic islands GIα and GIβ. Most strains of S. aureus possess a strong restriction barrier that hinders exchange of DNA. These RM systems present in S. aureus have limited functional genomic analysis to a small subset of strains that are amenable to genetic manipulation. The RM systems are classified into type I, II, and IV RM systems. (Monk and Foster 2012). The most widely distributed RM systems present and functional in majority of strains of S. aureus are the SauI type I RM system (Waldron and Lindsay 2006). Type I RM systems comprise genes that encode a host specificity of DNA (hsd) specificity (S) protein, a modification (M) protein, and restriction (R) endonuclease (Murray 2000). Type I RM systems protect self-DNA from cleavage by catalyzing methylation of hemi-methylated residues within a target sequence whilst also recognizing foreign unmethylated DNA at the same target sequence and cleaving DNA at a site distant to the target sequence (Waldron and Lindsay 2006). The type II RM system consists of DNA methylases and restriction endonuclease enzymes. There are at least 11 subclasses of type II RM enzymes, all of which recognize specific DNA sequences and digest the DNA at either specific points or a pre-determined distance away from the recognition site to yield restricted fragments. There are two type II RM enzymes in S. aureus, SauAI, which recognizes the double stranded GATC site and Sau961, which recognizes the DNA sequence GGNCC (Jones et al. 2015). However, these restriction endonuclease enzymes are only found in a small number of S. aureus strains (Stobberingh et al. 1977). The type IV systems is the simplest form of restriction system with a single protein able to detect the methylation status. The type IV restriction enzyme that recognizes cytosine methylated DNA has been shown to be the major barrier to transfer of plasmid DNA from Escherichia coli into Staphylococci.
Second, thickened cell wall peptidoglycan layers of the V036-V64 strain lowered the probability of electroporation. Optimization of conditions for generating competent cells for electroporation will be required to increase the frequency of transformation. However, the whole process of generating of mutants is a laborious and time-consuming process. To improve progress towards a more comprehensive understanding of S. aureus biology, the development of simple genetic manipulation methods for all S. aureus strains is necessary.
Telavancin is a semisynthetic lipoglycopeptide derivative of vancomycin. Telavancin has a glycopeptide core that binds with high affinity to the acyl-d-alanyl-d-alanine terminus of cell wall precursors through a network of hydrogen bonds and hydrophobic packing interactions. Thus, telavancin, like vancomycin, inhibits peptidoglycan by binding to late-stage peptidoglycan precursors. In this study, telavancin was observed to have a 23-fold higher MIC (0.064–1.5 μg/ml) against the V036-V64 strain than that against the V036 strain. This result is consistent with the previous reports, wherein telavancin showed high MIC against VISA isolates (Draghi et al. 2008; Karlowsky et al. 2015). Daptomycin interferes with bacterial cell membrane function (Howden et al. 2011). The MICs of this bacterial cell membrane-targeting lipopeptide antibiotic were ≤ 1 μg/ml and 4 μg/ml against the V036 and V036-V64 strains, respectively. This result is consistent with the previous report, wherein single-nucleotide substitutions within either walK or walR lead to co-resistance to vancomycin and daptomycin (Howden et al. 2011). These results suggest that the thickening of the cell wall decreases the interaction of cell wall-targeting antibiotics with peptidoglycan and its access to the cell membrane.
In conclusion, this study demonstrated that prolonged vancomycin exposure leads to the development of vancomycin resistance in MRSA strains. Compared with the vancomycin-susceptible parental strain, eight SNPs were detected in a high-level vancomycin-resistant strain. These genes are related with cell wall metabolism and growth defects. Description of new SNPs in the core genome, which can be linked with S. aureus highly resistance to vancomycin will allow development of better and more rapid diagnostic methods for identifying of drug resistance. It can also provide insights into the genetic basis of acquiring vancomycin resistance (apart from vanA gene acquisition) which are crucial for understanding and managing the emergence of new VRSA strains. In the road ahead, the relationships between the above gene mutations and resistance need to be analyzed to clarify the evolutionary pathways underpinning the development of high resistance to vancomycin.
References
Adegoke AA, Okoh AI (2014) Species diversity and antibiotic resistance properties of Staphylococcus aureus of farm animal origin in Nkonkobe Municipality, South Africa. Folia Microbiol (Praha) 59:133–140
Alam MT, Petit RA 3rd, Crispell EK, Thornton TA, Conneely KN, Jiang Y et al (2014) Dissecting vancomycin-intermediate resistance in Staphylococcus aureus using genome-wide association. Genome Biol Evol 6:1174–1185
Bae T, Schneewind O (2006) Allelic replacemnt in Staphylococcus aureus with inducible counter-selection. Plasmid 55:58–63
Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS et al (2012) SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19(5):455–477
Bhattacharyya D, Banerjee J, Bandyopadhyay S, Mondal B, Nanda PK et al (2016) First report on vancomycin-resistant Staphylococcus aureus in bovine and caprine milk. Microb Drug Resist 22(8):675–681
Boyle-Vavra S, Yin S, Jo DS, Montgomery CP, Daum RS (2013) VraT/YvqF is required for methicillin resistance and activation of the VraSR regulon in Staphylococcus aureus. Antimicrob Agents Chemother 57:83–95
Bylund GO, Lӧvgren JM, Wikstrӧm PM (2001) Characterization of mutations in the metY-nusA-infB operon that suppress the slow growth of a Δrim mutant. J Bacteriol 183:6095–6106
Cramer P, Bushnell DA, Kornberg RD (2001) Structural basis of transcription: RNA polymerase II at 2.8 angstrom resolution. Science 292:1863–1876
Delcher AL, Phillippy A, Carlton J, Salzberg SL (2002) Fast algorithms for largescale genome alignment and comparison. Nucleic Acids Res 30:2478–2483
Disz T, Akhter S, Cuevas D, Olson R, Overbeek R, Vonstein V, Stevens R et al (2010) Accessing the SEED genome database via web services API: tools for programmers. BMC Bioinform 11:319
Draghi DC, Benton BM, Krause K, Thornsberry C, Pillar C, Sahm DF (2008) In vitro activity of telavancin against recent gram-positive clinical isolates: results of the 2004–05 Prospective European Surveillance Initiative. J Antimicrob Chemother 62:116–121
Dubrac S, Boneca IG, Poupel O, Msadek T (2007) New insights into the WalK/WalR (YucG/YycF) essential signal transduction pathway reveal a major role in controlling cell wall metabolism and biofilm formation in Staphylococcus aureus. J Bacteriol 189:8257–8269
Gardete S, Kim C, Hartmann BM, Mwangi M, Roux CM, Dunman PM et al (2012) Genetic pathway in acquisition and loss of vancomycin resistance in a methicillin resistant Staphylococcus aureus (MRSA) strain of clonal type USA300. PLoS Pathog 8:e1002505
Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover FC (1997) Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother 40:135–136
Howden BP, Davies JK, Johnson PD, Stinear TP, Grayson ML (2010) Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: resistance mechansims, laboratory detection, and clinical implications. Clin Microbiol Rev 23:99–139
Howden BP, McEvoy CR, Allen DL, Chua K, Gao W, Harrison PF, Bell J et al (2011) Evolution of multidrug resistance drug Staphylococcus aureus infection involves mutation of the essential two component regulator WalKR. PLoS Pathog 7:e1002359
Howden BP, Peleg AY, Stinear TP (2014) The evolution of vancomycin intermediate Staphylococcus aureus (VISA) and heterogeneous-VISA. Infect Genet Evol 21:575–582
Hu Q, Peng H, Rao X (2016) Molecular events for promotion of vancomycin resistance in vancomycin intermediate Staphylococcus aureus. Front Microbiol 7:1601
Ishii K, Tabuchi F, Matsuo M, Tatsuno K, Sato T, Okazaki M et al (2015) Phenotypic and genomic comparisons of highly vancomycin-resistant Staphylococcus aureus strains developed from multiple clinical MRSA strains by in vitro mutagenesis. Sci Rep 5:17092
Jones MJ, Donegan NP, Mikheyeva IV, Cheung AL (2015) Improving transformation of Staphylococcus aureus belonging to the CC1, CC5 and CC8 clonal complexes. PLoS ONE 10(3):e0119487
Karlowsky JA, Nichol K, Zhanel GG (2015) Telavancin: mechanisms of action, in vitro activity, and mechanisms of resistance. Clin Infect Dis 61:S58–68
Kwok GM, O’Donoghue MM, Doddangoudar VC, Ho J, Boost MV (2013) Reduced vancomycin susceptibility in porcine ST9 MRSA isolates. Front Microbiol 4:316
Lӧvgren JM, Bylund GO, Srivastava MK, Lundberg LA, Persson OP et al (2004) The PRC-barrel domain of the ribosome maturation protein RimM mediates binding to ribosomal protein S19 in the 30S ribosomal subunits. RNA 10:1798–1812. https://doi.org/10.1261/rna.7720204
Monk IR, Foster TJ (2012) Genetic manipulation of Staphylococci-breaking through the barrier. Front Cell Infect Microbiol 2:49
Moreno LZ, Dutra MC, Moreno M, Ferreira TS, Silva GF, Matajira CE et al (2016) Vancomycin-intermediate livestock-associated methicillin-resistant Staphylococcus aureus ST398/t9358 from swine in Brazil. Mem Inst Oswaldo Cruz 111:659–661
Murray N (2000) Type I restriction system: sophisticated molecular machines (a legacy of Bertani and Weigle). Microbiol Mol Biol rev 64:412–434
Saito M, Katayama Y, Hishinuma T, Iwamoto A, Aiba Y, Kuwahara-Arai K et al (2014) “Slow VISA”, a novel phenotype of vancomycin resistance, found in vitro in heterogeneous vancomycin-intermediate Staphylococcus aureus strain Mu3. Antimicrob Agents Chemother 58:5024–5035
Shekarabi M, Hajikhani B, Salimi Chirani A, Fazeli M, Goudarzi M (2017) Molecular characterization of vancomycin-resistant Staphylococcus aureus strains isolated from clinical samples: a 3 year study in Tehran, Iran. PLoS ONE 12:e0183607
Sievert DM, Rudrick JT, Patel JB, McDonald LC, Wilkins MJ, Hageman JC (2008) Vancomycin-resistant Staphylococcus aureus in the United States, 2002–2006. Clin Infect Dis 46:668–674
Stobberingh E, Schiphof R, Sussenbach J (1977) Occurrence of a class II restriction endonuclease in Staphylococcus aureus. J Bacteriol 131:645–649
Takada H, Morita M, Shiwa Y, Sugimoto R, Suzuki S, Kawamura F, Yoshikawa H (2014) Cell motility and biofilm formation in Bacillus subtilis are affected by the ribosomal proteins, S11 and S21. Biosci Biotechnol Biochem 78:898–907
Tatusov RL, Koonin EV, Lipman DJ (1997) A genome perspective on protein families. Science 278:631–637
Tenover FC (2008) Vancomycin-resistant Staphylococcus aureus: perfect but geographically limited storm? Clin Infect Dis 46:675–677
Thati V, Shivannavar CT, Gaddad SM (2011) Vancomycin resistance among methicillin resistant Staphylococcus aureus isolates from intensive care units of tertiary care hospitals in Hyderabad. Indian J Med Res 134:704–708
Tiwari HK, Sen MR (2006) Emergence of vancomycin resistant Staphylococcus aureus (VRSA) from a tertiary care hospital from northern part of India. BMC Infect Dis 6:156
Waldron DE, Lindsay JA (2006) Sau1: a novel lineage-specific type I restriction-modification system that blocks horizontal gene transfer into Staphylococcus aureus and between S. aureus isolates of different lineages. J Bacteriol 188:5578–5585
Acknowledgements
This work was supported by an intramural research Grant awarded to the Korea Centers for Disease Control and Prevention (2016-NI44001-00).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, J.W., Lee, K.J. Single-nucleotide polymorphisms in a vancomycin-resistant Staphylococcus aureus strain based on whole-genome sequencing. Arch Microbiol 202, 2255–2261 (2020). https://doi.org/10.1007/s00203-020-01906-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-020-01906-y