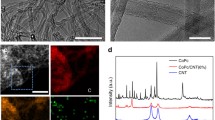
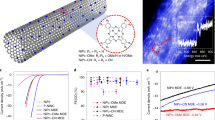
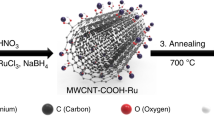
Abstract
Molecular electrocatalysts have demonstrated potential for the hydrogen evolution reaction (HER) due to their well-defined structures and high intrinsic activities. Achieving rapid production of hydrogen requires molecular electrocatalysts to operate at high current densities, which still presents a challenge. In this work, we demonstrate that molecularly dispersed electrocatalysts of cobalt phthalocyanine anchored on carbon nanotubes (CoPc MDEs) are superior candidates due to the efficient charge transport between the substrate and the active site. The intrinsic activity can be enhanced by introducing functional groups on phthalocyanine. To facilitate mass transport, di(ethylene glycol) substituted CoPc molecules are further anchored on a three-dimensional self-supported electrode (CoPc-DEG MDE@CC), enabling continuous operation for 25 h at −1000 mA/cm2 in 1.0 M KOH. Our study demonstrates the potential of molecular electrocatalysts for HER and emphasizes the importance of adjusting intrinsic activity, and charge and mass transport capacity for practical molecular electrocatalysts.

Similar content being viewed by others
References
Hu, H. S.; Wang, X. L.; Attfield, J. P.; Yang, M. H. Metal nitrides for seawater electrolysis. Chem. Soc. Rev. 2024, 53, 163–203.
Yang, H. Y.; Driess, M.; Menezes, P. W. Sdf-supooeted electrocatalysts for practical water electrolysis. Adv. Energy Mater. 2021, 11, 2102074.
Yan, D. F.; Mebrahtu, C.; Wang, S. Y.; Palkovits, R. Innovative electrochemical strategies for hydrogen production: From electricity input to electricity output. Angew. Chem., Int. Ed. 2023, 62, e202214333.
Cheng, R. Q.; Min, Y. L.; Li, H. X.; Fu, C. P. Electronic structure regulation in the design of low-cost efficient electrocatalysts: From theory to applications. Nano Energy 2023, 115, 108718.
Li, W.; Zhao, L.; Jiang, X. L.; Chen, Z. K.; Zhang, Y. G.; Wang, S. Y. Confinement engineering of electrocatalyst surfaces and interfaces. Adv. Funct. Mater. 2022, 32, 2207727.
Wu, H.; Huang, Q. X.; Shi, Y. Y.; Chang, J. W.; Lu, S. Y. Electrocatalytic water splitting: Mechanism and electrocatalyst design. Nano Res. 2023, 16, 9142–9157.
Cui, Z. B.; Jiao, W. S.; Huang, Z. Y.; Chen, G. Z.; Zhang, B.; Han, Y. H.; Huang, W. Design and synthesis of noble metal-based alloy electrocatalysts and their application in hydrogen evolution reaction. Small 2023, 19, 2301465.
Li, C. Q.; Kim, S. H.; Lim, H. Y.; Sun, Q. K.; Jiang, Y.; Noh, H. J.; Kim, S. J.; Baek, J.; Kwak, S. K.; Baek, J. B. Self-accommodation induced electronic metal-support interaction on ruthenium site for alkaline hydrogen evolution reaction. Adv. Mater. 2023, 35, 2301369.
Gong, F. L.; Liu, Y. H.; Zhao, Y.; Liu, W.; Zeng, G.; Wang, G. Q.; Zhang, Y. H.; Gong, L. H.; Liu, J. Universal sub-nanoreactor strategy for synthesis of yolk–shell MoS2 supported single atom electrocatalysts toward robust hydrogen evolution reaction. Angew. Chem., Int. Ed. 2023, 62, e202308091.
Quílez-Bermejo, J.; García-Dalí, S.; Daouli, A.; Zitolo, A.; Canevesi, R. L. S.; Emo, M.; Izquierdo, M. T.; Badawi, M.; Celzard, A.; Fierro, V. Advanced design of metal nanoclusters and single atoms embedded in C1N1-derived carbon materials for ORR, HER, and OER. Adv. Funct. Mater. 2023, 33, 2300405.
Zhang, F. M.; Liu, Y. L.; Yu, F.; Pang, H. J.; Zhou, X.; Li, D. Y.; Ma, W. Q.; Zhou, Q.; Mo, Y. X.; Zhou, H. Q. Engineering multilevel collaborative catalytic interfaces with multifunctional iron sites enabling high-performance real seawater splitting. ACS Nano 2023, 17, 1681–1692.
Zhang, L. L.; Wang, Z. P.; Zhang, J. T.; Lin, Z. P.; Zhang, Q. H.; Zhong, W. W.; Wu, G. F. High activity and stability in Ni2P/(Co, Ni)OOH heterointerface with a multiple-hierarchy structure for alkaline hydrogen evolution reaction. Nano Res. 2023, 16, 6552–6559.
Xie, L. S.; Tian, J.; Ouyang, Y. J.; Guo, X. A.; Zhang, W. A.; Apfel, U. P.; Zhang, W.; Cao, R. Water-soluble polymers with appending porphyrins as bioinspired catalysts for the hydrogen evolution reaction. Angew. Chem., Int. Ed. 2020, 59, 15844–15848.
Beyene, B. B.; Yibeltal, A. W.; Hung, C. H. Highly efficient electrocatalytic hydrogen evolution from neutral aqueous solution by water soluble copper(II) porphyrin. Inorg. Chim. Acta 2020, 513, 119929.
Yang, S. X.; Yu, Y. H.; Gao, X. J.; Zhang, Z. P.; Wang, F. Recent advances in electrocatalysis with phthalocyanines. Chem. Soc. Rev. 2021, 50, 12985–13011.
Dieng, M.; Contamin, O.; Savy, M. Problem of water electrolysis: Interest of catalysts containing molybdenum naphthalocyanines. Electrochim. Acta 1988, 33, 121–126.
Zagal, J. H.; Griveau, S.; Silva, J. F.; Nyokong, T.; Bedioui, F. Metallophthalocyanine-based molecular materials as catalysts for electrochemical reactions. Coordin. Chem. Rev. 2010, 554, 2755–2791.
Pegis, M. L.; Wise, C. F.; Martin, D. J.; Mayer, J. M. Oxygen reduction by homogeneous molecular catalysts and electrocatalysts. Chem. Rev. 2018, 118, 2340–2391.
Zhang, W.; Lai, W. Z.; Cao, R. Energy- related small molecule activation reactions: Oxygen reduction and hydrogen and oxygen evolution reactions catalyzed by porphyrin- and corrole-based systems. Chem. Rev. 2017, 117, 3717–3797.
Baglia, R. A.; Zaragoza, J. P. T.; Goldberg, D. P. Biomimetic reactivity of oxygen-derived manganese and iron porphyrinoid complexes. Chem. Rev. 2017, 117, 13320–13352.
Evans, D. J.; Pickett, C. J. Chemistry and the hydrogenases. Chem. Soc. Rev. 2003, 32, 268–275.
Li, X. L.; Lv, B.; Zhang, X. P.; Jin, X. T.; Guo, K.; Zhou, D. X.; Bian, H. T.; Zhang, W.; Apfel, U. P. et al. Introducing water-network-assisted proton transfer for boosted electrocatalytic hydrogen evolution with cobalt corrole. Angew. Chem., Int. Ed. 2022, 61, e202114310.
Wang, N.; Zhang, X. P.; Han, J. X.; Lei, H. T.; Zhang, Q. X.; Zhang, H.; Zhang, W.; Apfel, U. P.; Cao, R. Promoting hydrogen evolution reaction with a sulfonic proton relay. Chin. J. Catal. 2023, 45, 88–94.
Zhang, Q. X.; Lei, H. T.; Guo, H. B.; Wang, Y. B.; Gao, Y. M.; Zhang, W.; Cao, R. Through-space electrostatic effects of positively charged substituents on the hydrogen evolution reaction. ChemSusChem 2022, 15, e202200086.
Andreiadis, E. S.; Jacques, P. A.; Tran, P. D.; Leyris, A.; Chavarot-Kerlidou, M.; Jousselme, B.; Matheron, M.; Pécaut, J.; Palacin, S.; Fontecave, M. et al. Molecular engineering of a cobalt-based electrocatalytic nanomaterial for H2 evolution under fully aqueous conditions. Nat. Chem. 2013, 5, 48–53.
Li, X. L.; Lei, H. T.; Liu, J. Y.; Zhao, X. L.; Ding, S. P.; Zhang, Z. Y.; Tao, X. X.; Zhang, W.; Wang, W. C.; Zheng, X. H. et al. Carbon nanotubes with cobalt corroles for hydrogen and oxygen evolution in pH 0–14 solutions. Angew. Chem., Int. Ed. 2018, 130, 15290–15295.
Micheroni, D.; Lan, G. X.; Lin, W. B. Efficient electrocatalytic proton reduction with carbon nanotube-supported metal-organic frameworks. J. Am. Chem. Soc. 2018, 140, 15591–15595.
Zhang, B. Y.; Chen, L. L.; Zhang, Z. N.; Li, Q.; Khangale, P.; Hildebrandt, D.; Liu, X. Y.; Feng, Q. L.; Qiao, S. L. Modulating the band structure of metal coordinated salen COFs and an in situ constructed charge transfer heterostructure for electrocatalysis hydrogen evolution. Adv. Sci. 2022, 9, 2105912.
Seh, Z. W.; Kibsgaard, J.; Dickens, C. F.; Chorkendorff, I.; Nørskov, J. K.; Jaramillo, T. F. Combining theory and experiment in electrocatalysis: Insights into materials design. Scincee 2017, 355, eaad4998.
Zhang, Y. Y.; Chen, S. T.; Zhang, Y. X.; Li, R. J.; Zhao, B.; Peng, T. Y. Hydrogen-bond regulation of the microenvironment of Ni(II)-porphyrin bifunctional electrocatalysts for efficient overall water splitting. Adv. Mater. 2023, 35, 2210727.
Lin, L.; Li, H. B.; Yan, C. C.; Li, H. F.; Si, R.; Li, M. R.; Xiao, J. P.; Wang, G. X.; Bao, X. H. Synergistic catalysis over iron-nitrogen sites anchored with cobalt phthalocyanine for efficient CO2 electroreduction. Adv. Mater. 2019, 31, 1903470.
Zhang, X.; Wu, Z. S.; Zhang, X.; Li, L. W.; Li, Y. Y.; Xu, H. M.; Li, X. X.; Yu, X. L.; Zhang, Z. S.; Liang, Y. Y. et al. Highly selective and active CO2 reduction electrocatalysts based on cobalt phthalocyanine/carbon nanotube hybrid structures. Nat. Commun. 2017, 8, 14675.
Jiang, Z.; Zhang, Z. S.; Li, H.; Tang, Y. R.; Yuan, Y. B.; Zao, J.; Zheng, H. Z.; Liang, Y. Y. Molecular catalyst with near 100% selectivity for CO2 reduction in acidic electrolytes. Adv. Energy Mater. 2023, 13, 2203603.
Zhang, X.; Wang, Y.; Gu, M.; Wang, M. Y.; Zhang, Z. S.; Pan, W. Y.; Jiang, Z.; Zheng, H. Z.; Lucero, M.; Wang, H. L. et al. Molecular engineering of dispersed nickel phthalocyanines on carbon nanotubes for selective CO2 reduction. Nat. Energy 2020, 5, 684–692.
Lin, Z. C.; Jiang, Z.; Yuan, Y. B.; Li, H.; Wang, H. X.; Tang, Y. R.; Liu, C. C.; Liang, Y. Y. Cobalt-N4 macrocyclic complexes for heterogeneous electrocatalysis of the CO2 reduction reaction. Chin. J. Catal. 2022, 43, 104–109.
Fang, S.; Zhu, X. R.; Liu, X. K.; Gu, J.; Liu, W.; Wang, D. H.; Zhang, W.; Lin, Y.; Lu, J. L.; Wei, S. Q. et al. Uncovering near-free platinum single-atom dynamics during electrochemical hydrogen evolution reaction. Nat. Commun. 2020, 11, 1029.
Sun, T. T.; Zhao, S.; Chen, W. X.; Zhai, D.; Dong, J. C.; Wang, Y.; Zhang, S. L.; Han, A. J.; Gu, L.; Yu, R. et al. Single-atomic cobalt sites embedded in hierarchically ordered porous nitrogen-doped carbon as a superior bifunctional electrocatalyst. Proc. Natl. Acad. Sci. USA 2018, 115, 12692–12697.
Liang, H. W.; Brüller, S.; Dong, R. H.; Zhang, J.; Feng, X. L.; Müllen, K. Molecular metal-Nx centres in porous carbon for electrocatalytic hydrogen evolution. Nat. Commun. 2015, 6, 7992.
Fei, H. L.; Dong, J. C.; Arellano-Jiménez, M. J.; Ye, G. L.; Kim, N. D.; Samuel, E. L. G.; Peng, Z. W.; Zhu, Z.; Qin, F.; Bao, J. M. et al. Atomic cobalt on nitrogen-doped graphene for hydrogen generation. Nat. Commun. 2015, 6, 8668.
Liu, R.; Gong, Z. C.; Liu, J. B.; Dong, J. C.; Liao, J. W.; Liu, H.; Huang, H. K.; Liu, J. J.; Yan, M. M.; Huang, K. et al. Design of aligned porous carbon films with single-atom Co-N-C sites for high-current-density hydrogen generation. Adv. Mater. 2021, 33, 2103533.
Chen, B. T.; Zou, H. Y.; Gong, L.; Zhang, H.; Li, N.; Pan, H. H.; Wang, K.; Yang, T.; Liu, Y. P.; Duan, L. L. et al. Molecular engineering of dispersed tin phthalocyanine on carbon nanotubes for selective CO2 reduction to formate. Appl. Catal. B 2024, 344, 123650.
Wu, Y. S.; Jiang, Z.; Lu, X.; Liang, Y. Y.; Wang, H. L. Domino electroreduction of CO2 to methanol on a molecular catalyst. Nature 2019, 575, 639–642.
Su, J. J.; Zhang, J. J.; Chen, J. C.; Song, Y.; Huang, L. B.; Zhu, M. H.; Yakobson, B. I.; Tang, B. Z.; Ye, R. Q. Building a stable cationic molecule/electrode interface for highly efficient and durable CO2 reduction at an industrially relevant current. Energy Environ. Sci. 2021, 14, 483–492.
Zhu, W. W.; Liu, S. Q.; Zhao, K. M.; Ye, G. Y.; Huang, K.; He, Z. Revealing a double-volcano-like structure-activity relationship for substitution-functionalized metal-phthalocyanine catalysts toward electrochemical CO2 reduction. Small 2024, 20, 2306144.
Chen, K. J.; Cao, M. Q.; Lin, Y. Y.; Fu, J. W.; Liao, H. X.; Zhou, Y. J.; Li, H. M.; Qiu, X. Q.; Hu, J. H.; Zheng, X. S. et al. Ligand engineering in nickel phthalocyanine to boost the electrocatalytic reduction of CO2. Adv. Funct. Mater. 2022, 32, 2111322.
Yuan, S.; Peng, J. Y.; Zhang, Y. R.; Zheng, D. J.; Bagi, S.; Wang, T.; Román-Leshkov, Y.; Shao-Horn, Y. Tuning the catalytic activity of Fe-phthalocyanine-based catalysts for the oxygen reduction reaction by ligand functionalization. ACS Catal. 2022, 12, 7278–7287.
Wang, Y.; Zhang, Z. S.; Zhang, X.; Yuan, Y. B.; Jiang, Z.; Zheng, H. Z.; Wang, Y. G.; Zhou, H.; Liang, Y. Y. Theory-driven design of electrocatalysts for the two-electron oxygen reduction reaction based on dispersed metal phthalocyanines. CCS Chem. 2022, 4, 228–236.
Mu, X. Q.; Gu, X. Y.; Dai, S. P.; Chen, J. B.; Cui, Y. J.; Chen, Q.; Yu, M.; Chen, C. Y.; Liu, S. L.; Mu, S. C. Breaking the symmetry of single-atom catalysts enables an extremely low energy barrier and high stability for large-current-density water splitting. Energy Environ. Sci. 2022, 15, 4048–4057.
Wang, A. J.; Li, C.; Zhang, J.; Chen, X. D.; Cheng, L. X.; Zhu, W. H. Graphene-oxide- supported covalent organic polymers based on zinc phthalocyanine for efficient optical limiting and hydrogen evolution. J. Colloid Interf. Sci. 2019, 556, 159–171.
Gu, T. T.; Attatsi, I. K.; Zhu, W. H.; Li, M. Z.; Ndur, S. A.; Liang, X. Enhanced electrocatalytic hydrogen evolutions of Co(II)phthalocyanine through axially coordinated pyridine-pyrene. Inorg. Chim. Acta 2022, 530, 120696.
Xu, G. L.; Lei, H. T.; Zhou, G. J.; Zhang, C. C.; Xie, L. S.; Zhang, W.; Cao, R. Boosting hydrogen evolution by using covalent frameworks of fluorinated cobalt porphyrins supported on carbon nanotubes. Chem. Commun. 2019, 55, 12647–12650.
Acknowledgements
This work was supported by Guangdong-Hong Kong-Macao Joint Laboratory for Photonic-Thermal-Electrical Energy Materials and Devices (No. 2019B121205001), Shenzhen fundamental research funding (Nos. JCYJ20220818100618039 and JCYJ20200109141405950), and the National Natural Science Foundation of China (No. 22075125). The computational resource was supported by the Center for Computational Science and Engineering (SUSTech).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Li, H., Jiang, Z., Yuan, Y. et al. Multiscale engineering of molecular electrocatalysts for the rapid hydrogen evolution reaction. Nano Res. (2024). https://doi.org/10.1007/s12274-024-6660-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12274-024-6660-z