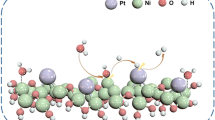
Abstract
The reduction degree of TiO2 support is critical to the performances of metal catalysts. In many previous theoretical calculations, only the bridge oxygen vacancy (Ov) was considered as the electron-donating defect on reduced rutile TiO2 (r-TiO2−x) supports. However, titanium adatoms (Tiad.), oxidized titanium islands (Tiad.On), and acid hydroxyls (ObrH) also exist at the metal/support interface. By conducting density functional theory (DFT) calculations and ab initio molecular dynamics (AIMD) simulations, we compared r-TiO2−x surfaces with Ov, Tiad., Tiad.On, and ObrH sites loaded with Au nanoparticles (NPs). The results showed the Au NPs were oxygen-phobic but titanium-philic, resulting in wetting of Ov and Tiad. but short contact with Tiad.On and ObrH. The Bader charges of Au NPs (QM) showed a good linear relationship with the ideal number of donating electrons (Ne) from the defective sites (QM = −KeNe + QM,S), demonstrating the intrinsic electron allocation at the interface. The Ov, Tiad., and Tiad.On exhibited similar slopes (Ke), relatively steeper than that of ObrH. That means in the scope of Au NP charge state, the Tiad. and Tiad.On have a close electron-donating ability with Ov, but the ObrH donates relatively fewer electrons. This linear relationship can be extended approximately to other metals. The higher the metal work function, the steeper the Ke for easier electron donation from defective sites. The stronger the metal oxygen affinity, the more positive the intercept (QM,S). That explains the easy generation of metallic or negative Pt and Au NPs on r-TiO2−x, but hard for Cu and Zn in experiment. That provides theoretical guidance for regulating the charge of metal NPs over TiO2−x supports.

Similar content being viewed by others
References
Mehrabadi, B. A. T.; Eskandari, S.; Khan, U.; White, R. D.; Regalbuto, J. R. Chapter One—A review of preparation methods for supported metal catalysts. Adv. Catal. 2017, 61, 1–35.
Ndolomingo, M. J.; Bingwa, N.; Meijboom, R. Review of supported metal nanoparticles: Synthesis methodologies, advantages and application as catalysts. J. Mater. Sci. 2020, 55, 6195–6241.
Wang, H. W.; Lu, J. L. A review on particle size effect in metal-catalyzed heterogeneous reactions. Chin. J. Chem. 2020, 38, 1422–1444.
Liu, L. C.; Corma, A. Metal catalysts for heterogeneous catalysis: From single atoms to nanoclusters and nanoparticles. Chem. Rev. 2018, 118, 4981–5079.
van Deelen, T. W.; Hernández Mejía, C.; de Jong, K. P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2019, 2, 955–970.
Li, Y. Y.; Zhang, Y. S.; Qian, K.; Huang, W. X. Metal-support interactions in metal/oxide catalysts and oxide-metal interactions in oxide/metal inverse catalysts. ACS Catal. 2022, 12, 1268–1287.
Sadakiyo, M. Support effects of metal-organic frameworks in heterogeneous catalysis. Nanoscale 2022, 14, 3398–3406.
Gerber, I. C.; Serp, P. A theory/experience description of support effects in carbon-supported catalysts. Chem. Rev. 2020, 120, 1250–1349.
Hua, M. L.; Song, J. L.; Huang, X.; Hou, M. Q.; Fan, H. L.; Zhang, Z. F.; Wu, T. B.; Han, B. X. Support effect of Ru catalysts for efficient conversion of biomass-derived 2,5-hexanedione to different products. ACS Catal. 2021, 11, 7685–7693.
Comotti, M.; Li, W. C.; Spliethoff, B.; Schüth, F. Support effect in high activity gold catalysts for CO oxidation. J. Am. Chem. Soc. 2006, 128, 917–924.
Hernández Mejía, C.; van Deelen, T. W.; de Jong, K. P. Activity enhancement of cobalt catalysts by tuning metal-support interactions. Nat. Commun. 2018, 9, 4459.
Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and catalytic applications of CeO2-based materials. Chem. Rev. 2016, 116, 5987–6041.
Valden, M.; Lai, X.; Goodman, D. W. Onset of catalytic activity of gold clusters on Titania with the appearance of nonmetallic properties. Science 1998, 281, 1647–1650.
Janssens, T. V. W.; Clausen, B. S.; Hvolbæk, B.; Falsig, H.; Christensen, C. H.; Bligaard, T.; Nørskov, J. K. Insights into the reactivity of supported Au nanoparticles: Combining theory and experiments. Top. Catal. 2007, 44, 15–26.
Overbury, S. H.; Schwartz, V.; Mullins, D. R.; Yan, W.; Dai, S. Evaluation of the Au size effect: CO oxidation catalyzed by Au/TiO2. J. Catal. 2006, 241, 56–65.
Kung, H. H.; Kung, M. C.; Costello, C. K. Supported Au catalysts for low temperature CO oxidation. J. Catal. 2003, 216, 425–432.
Schlexer, P.; Widmann, D.; Behm, R. J.; Pacchioni, G. CO oxidation on a Au/TiO2 nanoparticle catalyst via the Au-assisted Mars–van Krevelen mechanism. ACS Catal. 2018, 8, 6513–6525.
Guan, X. J.; Liu, M. C.; Mao, S. S.; Shen, S. H. Enhanced photocatalytic water splitting of TiO2 by decorating with facet-controlled Au nanocrystals. Appl. Phys. Lett. 2021, 119, 143901.
Tanaka, A.; Teramura, K.; Hosokawa, S.; Kominami, H.; Tanaka, T. Visible light-induced water splitting in an aqueous suspension of a plasmonic Au/TiO2 photocatalyst with metal co-catalysts. Chem. Sci. 2017, 8, 2574–2580.
Rayalu, S. S.; Jose, D.; Joshi, M. V.; Mangrulkar, P. A.; Shrestha, K.; Klabunde, K. Photocatalytic water splitting on Au/TiO2 nanocomposites synthesized through various routes: Enhancement in photocatalytic activity due to SPR effect. Appl. Catal. B: Environ. 2013, 142–143, 684–693.
Goodman, D. W. “Catalytically active Au on Titania”: Yet another example of a strong metal support interaction (SMSI)? Catal. Lett. 2005, 99, 1–4.
Wang, Z. H.; Fu, H. F.; Tian, Z. W.; Han, D. M.; Gu, F. B. Strong metal-support interaction in novel core-shell Au-CeO2 nanostructures induced by different pretreatment atmospheres and its influence on CO oxidation. Nanoscale 2016, 8, 5865–5872.
Zhang, Y. S.; Liu, J. X.; Qian, K.; Jia, A. P.; Li, D.; Shi, L.; Hu, J.; Zhu, J. F.; Huang, W. X. Structure sensitivity of Au-TiO2 strong metal-support interactions. Angew. Chem., Int. Ed. 2021, 60, 12074–12081.
Du, X. R.; Huang, Y. K.; Pan, X. L.; Han, B.; Su, Y.; Jiang, Q. K.; Li, M. R.; Tang, H. L.; Li, G.; Qiao, B. T. Size- dependent strong metal-support interaction in TiO2 supported Au nanocatalysts. Nat. Commun. 2020, 11, 5811.
Tang, H. L.; Su, Y.; Zhang, B. S.; Lee, A. F.; Isaacs, M. A.; Wilson, K.; Li, L.; Ren, Y. G.; Huang, J. H.; Haruta, M. et al. Classical strong metal-support interactions between gold nanoparticles and titanium dioxide. Sci. Adv. 2017, 3, e1700231.
Hvolbæk, B.; Janssens, T. V. W.; Clausen, B. S.; Falsig, H.; Christensen, C. H.; Nørskov, J. K. Catalytic activity of Au nanoparticles. Nano Today 2007, 2, 14–18.
Saqlain, M. A.; Hussain, A.; Siddiq, M.; Ferreira, A. R.; Leitão, A. A. Thermally activated surface oxygen defects at the perimeter of Au/TiO2: A DFT+U study. Phys. Chem. Chem. Phys. 2015, 17, 25403–25410.
Saqlain, M. A.; Novais Antunes, F. P.; Hussain, A.; Siddiq, M.; Leitão, A. A. Adsorption of oxygen and CO oxidation on Au/anatase (001) catalysts. A DFT+U study. New J. Chem. 2017, 41, 2073–2080.
Saqlain, M. A.; Hussain, A.; Siddiq, M.; Leitão, A. A. A DFT+U study of the mars van Krevelen mechanism of CO oxidation on Au/TiO2 catalysts. Appl. Catal. A: Gen. 2016, 519, 27–33.
Chen, Y.; Crawford, P.; Hu, P. Recent advances in understanding CO oxidation on gold nanoparticles using density functional theory. Catal. Lett. 2007, 119, 21–28.
Remediakis, I. N.; Lopez, N.; Nørskov, J. K. CO oxidation on rutile-supported Au nanoparticles. Angew. Chem., Int. Ed. 2005, 44, 1824–1826.
Tauster, S. J. Strong metal-support interactions. Acc. Chem. Res. 1987, 20, 389–394.
Fu, Q.; Wagner, T.; Olliges, S.; Carstanjen, H. D. Metal-oxide interfacial reactions: Encapsulation of Pd on TiO2 (110). J. Phys. Chem. B 2005, 109, 944–951.
Fang, W. Z.; Xing, M. Y.; Zhang, J. L. Modifications on reduced titanium dioxide photocatalysts: A review. J. Photochem. Photobiol. C: Photochem. Rev. 2017, 32, 21–39.
Zhang, J. L.; Tian, B. Z.; Wang, L. Z.; Xing, M. Y.; Lei, J. Y. Mechanism of photocatalysis. In Photocatalysis: Fundamentals, Materials and Applications, Zhang, J. L.; Tian, B. Z.; Wang, L. Z.; Xing, M. Y.; Lei, J. Y., Eds.; Springer: Singapore, 2018; pp 1–15.
Henderson, M. A. An HREELS and TPD study of water on TiO2 (110): The extent of molecular versus dissociative adsorption. Surf. Sci. 1996, 355, 151–166.
Pan, J. M.; Maschhoff, B. L.; Diebold, U.; Madey, T. E. Interaction of water, oxygen, and hydrogen with TiO2 (110) surfaces having different defect densities. J. Vac. Sci. Technol. A 1992, 10, 2470–2476.
Geng, Z. H.; Jin, X. C.; Wang, R. M.; Chen, X.; Guo, Q.; Ma, Z. B.; Dai, D. X.; Fan, H. J.; Yang, X. M. Low- temperature hydrogen production via water conversion on Pt/TiO2. J. Phys. Chem. C 2018, 122, 10956–10962.
Bennett, R. A.; McCavish, N. D. Non- stoichiometric oxide surfaces and ultra-thin films: Characterisation of TiO2. Top. Catal. 2005, 36, 11–19.
Sanville, E. J.; Vernon, L. J.; Kenny, S. D.; Smith, R.; Moghaddam, Y.; Browne, C.; Mulheran, P. Surface and interstitial transition barriers in rutile (110) surface growth. Phys. Rev. B 2009, 80, 235308.
Mulheran, P. A.; Nolan, M.; Browne, C. S.; Basham, M.; Sanville, E.; Bennett, R. A. Surface and interstitial Ti diffusion at the rutile TiO2 (110) surface. Phys. Chem. Chem. Phys. 2010, 12, 9763–9771.
Finazzi, E.; Di Valentin, C.; Pacchioni, G. Nature of Ti interstitials in reduced bulk anatase and rutile TiO2. J. Phys. Chem. C 2009, 113, 3382–3385.
Wendt, S.; Schaub, R.; Matthiesen, J.; Vestergaard, E. K.; Wahlström, E.; Rasmussen, M. D.; Thostrup, P.; Molina, L. M.; Lægsgaard, E.; Stensgaard, I. et al. Oxygen vacancies on TiO2 and their interaction with H2O and O2: A combined high-resolution STM and DFT study. Surf. Sci. 2005, 598, 226–245.
Hammer, B.; Wendt, S.; Besenbacher, F. Water adsorption on TiO2. Top. Catal. 2010, 53, 423–430.
Petrik, N. G.; Kimmel, G. A. Reaction kinetics of water molecules with oxygen vacancies on rutile TiO2 (110). J. Phys. Chem. C 2015, 119, 23059–23067.
Wang, Y. G.; Yoon, Y.; Glezakou, V. A.; Li, J.; Rousseau, R. The role of reducible oxide-metal cluster charge transfer in catalytic processes: New insights on the catalytic mechanism of CO oxidation on Au/TiO2 from ab initio molecular dynamics. J. Am. Chem. Soc. 2013, 135, 10673–10683.
Wang, Y. G.; Cantu, D. C.; Lee, M. S.; Li, J.; Glezakou, V. A.; Rousseau, R. CO oxidation on Au/TiO2: Condition-dependent active sites and mechanistic pathways. J. Am. Chem. Soc. 2016, 138, 10467–10476.
Cao, W.; Xia, G. J.; Yao, Z.; Zeng, K. H.; Qiao, Y.; Wang, Y. G. Aldehyde hydrogenation by Pt/TiO2 catalyst in aqueous phase: Synergistic effect of oxygen vacancy and solvent water. JACS Au 2023, 3, 143–153.
Petrik, N. G.; Zhang, Z. R.; Du, Y. G.; Dohnálek, Z.; Lyubinetsky, I.; Kimmel, G. A. Chemical reactivity of reduced TiO2 (110): The dominant role of surface defects in oxygen chemisorption. J. Phys. Chem. C 2009, 113, 12407–12411.
Wahlström, E.; Lopez, N.; Schaub, R.; Thostrup, P.; Rennau, A.; Africh, C.; Lægsgaard, E.; Nørskov, J. K.; Besenbacher, F. Bonding of gold nanoclusters to oxygen vacancies on rutile TiO2 (110). Phys. Rev. Lett. 2003, 90, 026101.
Galhenage, R. P.; Yan, H.; Tenney, S. A.; Park, N.; Henkelman, G.; Albrecht, P.; Mullins, D. R.; Chen, D. A. Understanding the nucleation and growth of metals on TiO2: Co compared to Au, Ni, and Pt. J. Phys. Chem. C 2013, 117, 7191–7201.
Agacino Valdés, E.; Tavizón, G.; de la Mora, P. Theoretical study of Aun clusters (n = 1–5) deposited on a rutile TiO2 (110) slab, concerning structure and stability. J. Comput. Chem. 2020, 41, 2750–2757.
Xia, G. J.; Lee, M. S.; Glezakou, V. A.; Rousseau, R.; Wang, Y. G. Diffusion and surface segregation of interstitial Ti defects induced by electronic metal-support interactions on a Au/TiO2 nanocatalyst. ACS Catal. 2022, 12, 4455–4464.
Tanaka, T.; Sumiya, A.; Sawada, H.; Kondo, Y.; Takayanagi, K. Direct observation of interstitial titanium ions in TiO2 substrate with gold nanoparticle. Surf. Sci. 2014, 619, 39–43.
Bennett, R. A.; Stone, P.; Bowker, M. Pd nanoparticle enhanced Re-oxidation of non-stoichiometric TiO2: STM imaging of spillover and a new form of SMSI. Catal. Lett. 1999, 59, 99–105.
Wan, W. J.; Nie, X. W.; Janik, M. J.; Song, C. S.; Guo, X. W. Adsorption, dissociation, and spillover of hydrogen over Au/TiO2 catalysts: The effects of cluster size and metal-support interaction from DFT. J. Phys. Chem. C 2018, 122, 17895–17916.
Zhu, Y. M.; Liu, D. S.; Meng, M. H2 spillover enhanced hydrogenation capability of TiO2 used for photocatalytic splitting of water: A traditional phenomenon for new applications. Chem. Commun. 2014, 50, 6049–6051
Panayotov, D. A.; Burrows, S. P.; Yates, J. T.; Morris, J. R. Mechanistic studies of hydrogen dissociation and spillover on Au/TiO2: IR spectroscopy of coadsorbed CO and H-donated electrons. J. Phys. Chem. C 2011, 115, 22400–22408.
Mori, K.; Hashimoto, N.; Kamiuchi, N.; Yoshida, H.; Kobayashi, H.; Yamashita, H. Hydrogen spillover-driven synthesis of high-entropy alloy nanoparticles as a robust catalyst for CO2 hydrogenation. Nat. Commun. 2021, 12, 3884.
Sravan Kumar, K. B.; Whittaker, T. N.; Peterson, C.; Grabow, L. C.; Chandler, B. D. Water poisons H2 activation at the Au-TiO2 interface by slowing proton and electron transfer between Au and Titania. J. Am. Chem. Soc. 2020, 142, 5760–5772.
Lykhach, Y.; Kozlov, S. M.; Skála, T.; Tovt, A.; Stetsovych, V.; Tsud, N.; Dvořák, F.; Johánek, V.; Neitzel, A.; Mysliveček, J. et al. Counting electrons on supported nanoparticles. Nat. Mater. 2016, 15, 284–288.
Aso, R.; Hojo, H.; Takahashi, Y.; Akashi, T.; Midoh, Y.; Ichihashi, F.; Nakajima, H.; Tamaoka, T.; Yubuta, K.; Nakanishi, H. et al. Direct identification of the charge state in a single platinum nanoparticle on titanium oxide. Science 2022, 378, 202–206.
Kühne, T. D.; Iannuzzi, M.; Del Ben, M.; Rybkin, V. V.; Seewald, P.; Stein, F.; Laino, T.; Khaliullin, R. Z.; Schütt, O.; Schiffmann, F. et al. CP2K: An electronic structure and molecular dynamics software package-quickstep: Efficient and accurate electronic structure calculations. J. Chem. Phys. 2020, 152, 194103.
Perdew, J. P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1997, 78, 1396.
Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104.
Farnesi Camellone, M.; Marx, D. On the impact of solvation on a Au/TiO2 nanocatalyst in contact with water. J. Phys. Chem. Lett. 2013, 4, 514–518.
Lu, Y.; Yin, W. J.; Peng, K. L.; Wang, K.; Hu, Q.; Selloni, A.; Chen, F. R.; Liu, L. M.; Sui, M. L. Self- hydrogenated shell promoting photocatalytic H2 evolution on anatase TiO2. Nat. Commun. 2018, 9, 2752.
Ji, Y. F.; Wang, B.; Luo, Y. Location of trapped hole on rutile-TiO2 (110) surface and its role in water oxidation. J. Phys. Chem. C 2012, 116, 7863–7866.
Setvin, M.; Franchini, C.; Hao, X. F.; Schmid, M.; Janotti, A.; Kaltak, M.; Van De Walle, C. G.; Kresse, G.; Diebold, U. Direct view at excess electrons in TiO2 rutile and anatase. Phys. Rev. Lett. 2014, 113, 086402.
Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519.
Whittaker, T.; Kumar, K. B. S.; Peterson, C.; Pollock, M. N.; Grabow, L. C.; Chandler, B. D. H2 oxidation over supported Au nanoparticle catalysts: Evidence for heterolytic H2 activation at the metal-support interface. J. Am. Chem. Soc. 2018, 140, 16469–16487.
Lemire, C.; Meyer, R.; Shaikhutdinov, S.; Freund, H. J. Do quantum size effects control CO adsorption on gold nanoparticles. Angew. Chem., Int. Ed. 2004, 43, 118–121.
Mavrikakis, M.; Stoltze, P.; Nørskov, J. K. Making gold less noble. Catal. Lett. 2000, 64, 101–106.
Drummond, T. J. Work functions of the transition metals and metal silicides. J. Appl. Phys. 1999, 85, 1–24.
Bard, A. J.; Parsons, R.; Jordan, J. Standard Potentials in Aqueous Solution; CRC Press: New York, 2017.
An, K.; Somorjai, G. A. Size and shape control of metal nanoparticles for reaction selectivity in catalysis. ChemCatChem 2012, 4, 1512–1524.
Lin, L. N.; Zhong, Q. L.; Zheng, Y. Z.; Cheng, Y.; Qi, R. J.; Huang, R. Size effect of Au nanoparticles in Au-TiO2−x photocatalyst. Chem. Phys. Lett. 2021, 770, 138457.
Yogi, C.; Kojima, K.; Hashishin, T.; Wada, N.; Inada, Y.; Della Gaspera, E.; Bersani, M.; Martucci, A.; Liu, L. J.; Sham, T. K. Size effect of Au nanoparticles on TiO2 crystalline phase of nanocomposite thin films and their photocatalytic properties. J. Phys. Chem. C 2011, 115, 6554–6560.
Kuo, C. T.; Lu, Y. B.; Kovarik, L.; Engelhard, M.; Karim, A. M. Structure sensitivity of acetylene semi-hydrogenation on Pt single atoms and subnanometer clusters. ACS Catal. 2019, 9, 11030–11041.
Wang, W. N.; An, W. J.; Ramalingam, B.; Mukherjee, S.; Niedzwiedzki, D. M.; Gangopadhyay, S.; Biswas, P. Size and structure matter: Enhanced CO2 photoreduction efficiency by size-resolved ultrafine Pt nanoparticles on TiO2 single crystals. J. Am. Chem. Soc. 2012, 134, 11276–11281.
Dessal, C.; Martínez, L.; Maheu, C.; Len, T.; Morfin, F.; Rousset, J. L.; Puzenat, E.; Afanasiev, P.; Aouine, M.; Soler, L. et al. Influence of Pt particle size and reaction phase on the photocatalytic performances of ultradispersed Pt/TiO2 catalysts for hydrogen evolution. J. Catal. 2019, 375, 155–163.
Vajda, S.; Pellin, M. J.; Greeley, J. P.; Marshall, C. L.; Curtiss, L. A.; Ballentine, G. A.; Elam, J. W.; Catillon-Mucherie, S.; Redfern, P. C.; Mehmood, F. et al. Subnanometre platinum clusters as highly active and selective catalysts for the oxidative dehydrogenation of propane. Nat. Mater. 2009, 8, 213–216.
Beck, A.; Huang, X.; Artiglia, L.; Zabilskiy, M.; Wang, X.; Rzepka, P.; Palagin, D.; Willinger, M. G.; van Bokhoven, J. A. The dynamics of overlayer formation on catalyst nanoparticles and strong metal-support interaction. Nat. Commun. 2020, 11, 3220.
Frey, H.; Beck, A.; Huang, X.; Van Bokhoven, J. A.; Willinger, M. G. Dynamic interplay between metal nanoparticles and oxide support under redox conditions. Science 2022, 376, 982–987.
Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (No. 2022YFA1503102), the National Natural Science Foundation of China (Nos. 22022504, 22003022, and 22203041), Guangdong Basic and Applied Basic Research Foundation, China (No. 2021A1515110406), Guangdong “Pearl River” Talent Plan (No. 2019QN01L353), and Guangdong Provincial Key Laboratory of Catalysis (No. 2020B121201002). Most calculations were performed on the CHEM high-performance computing cluster (CHEM-HPC) located at the Department of Chemistry, SUSTech. The computational resources were also supported by the Center for Computational Science and Engineering at the Southern University of Science and Technology (SUSTech).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Xia, GJ., Fu, Y., Cao, W. et al. How interfacial electron-donating defects influence the structure and charge of gold nanoparticles on TiO2 support. Nano Res. (2024). https://doi.org/10.1007/s12274-024-6625-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12274-024-6625-2