Abstract
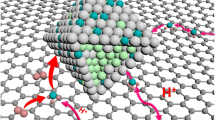
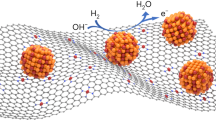
The sluggish reaction kinetics of alkaline hydrogen oxidation reaction (HOR) is one of the key challenges for anion exchange membrane fuel cells (AEMFCs). To achieve robust alkaline HOR with minimized cost, we developed a single atom-cluster multiscale structure with isolated Pt single atoms anchored on Ru nanoclusters supported on nitrogen-doped carbon nanosheets (Pt1-Ru/NC). The well-defined structure not only provides multiple sites with varied affinity with the intermediates but also enables simultaneous modulation of different sites via interfacial interaction. In addition to weakening Ru–H bond strength, the isolated Pt sites are heavily involved in hydrogen adsorption and synergistically accelerate the Volmer step with the help of Ru sites. Furthermore, this catalyst configuration inhibits the excessive occupancy of oxygen-containing species on Ru sites and facilitates the HOR at elevated potentials. The Pt1-Ru/NC catalyst exhibits superior alkaline HOR performance with extremely high activity and excellent CO-tolerance. An AEMFC with a 0.1 mg·cmPGM−2 loading of Pt1-Ru/NC anode catalyst achieves a peak powder density of 1172 mW·cm−2, which is 2.17 and 1.55 times higher than that of Pt/C and PtRu/C, respectively. This work provides a new catalyst concept to address the sluggish kinetics of electrocatalytic reactions containing multiple intermediates and elemental steps.

Similar content being viewed by others
References
Strmcnik, D.; Uchimura, M.; Wang, C.; Subbaraman, R.; Danilovic, N.; van der Vliet, D.; Paulikas, A. P.; Stamenkovic, V. R.; Markovic, N. M. Improving the hydrogen oxidation reaction rate by promotion of hydroxyl adsorption. Nat. Chem. 2013, 5, 300–306.
Yao, Z. C.; Tang, T.; Jiang, Z.; Wang, L.; Hu, J. S.; Wan, L. J. Electrocatalytic hydrogen oxidation in alkaline media: From mechanistic insights to catalyst design. ACS Nano 2022, 16, 5153–5183.
Xiao, F.; Wang, Y. C.; Wu, Z. P.; Chen, G. Y.; Yang, F.; Zhu, S. Q.; Siddharth, K.; Kong, Z. J.; Lu, A. L.; Li, J. C. et al. Recent advances in electrocatalysts for proton exchange membrane fuel cells and alkaline membrane fuel cells. Adv. Mater. 2021, 33, 2006292.
Wang, Y. D.; Meyer, Q.; Tang, K. N.; McClure, J. E.; White, R. T.; Kelly, S. T.; Crawford, M. M.; Iacoviello, F.; Brett, D. J. L.; Shearing, P. R. et al. Large-scale physically accurate modelling of real proton exchange membrane fuel cell with deep learning. Nat. Commun. 2023, 14, 745.
Pan, Z. F.; An, L.; Zhao, T. S.; Tang, Z. K. Advances and challenges in alkaline anion exchange membrane fuel cells. Prog. Energy Combust. Sci. 2018, 66, 141–175.
Zhao, R. P.; Yue, X.; Li, Q. H.; Fu, G. T.; Lee, J. M.; Huang, S. M. Recent advances in electrocatalysts for alkaline hydrogen oxidation reaction. Small 2021, 17, 2100391.
Zhuang, Z. C.; Xia, L. X.; Huang, J. Z.; Zhu, P.; Li, Y.; Ye, C. L.; Xia, M. G.; Yu, R. H.; Lang, Z. Q.; Zhu, J. X. et al. Continuous modulation of electrocatalytic oxygen reduction activities of single-atom catalysts through p-n junction rectification. Angew. Chem., Int. Ed. 2023, 62, e202212335.
Yang, Z. L.; Lai, W. C.; He, B. L.; Wang, J.; Yu, F. F.; Liu, Q. H.; Liu, M. C.; Zhang, S. G.; Ding, W.; Lin, Z. Q. et al. Tailoring interfacial chemistry of defective carbon-supported Ru catalyst toward efficient and co-tolerant alkaline hydrogen oxidation reaction. Adv. Energy Mater. 2023, 13, 2300881.
Gan, T.; Wang, D. S. Atomically dispersed materials: Ideal catalysts in atomic era. Nano Res. 2024, 17, 18–38.
Wang, L. G.; Wu, J. B.; Wang, S. W.; Liu, H.; Wang, Y.; Wang, D. S. The reformation of catalyst: From a trial-and-error synthesis to rational design. Nano Res. 2024, 17, 3261–3301.
Zhu, C. X.; Yang, J. R.; Zhang, J. W.; Wang, X. Q.; Gao, Y.; Wang, D. S.; Pan, H. G. Single-atom materials: The application in energy conversion. Int. Mater. 2024, 3, 74–86.
Lu, S. Q.; Zhuang, Z. B. Investigating the influences of the adsorbed species on catalytic activity for hydrogen oxidation reaction in alkaline electrolyte. J. Am. Chem. Soc. 2017, 139, 5156–5163.
Ma, M.; Li, G.; Yan, W.; Wu, Z. Z.; Zheng, Z. P.; Zhang, X. B.; Wang, Q. X.; Du, G. F.; Liu, D. Y.; Xie, Z. X. et al. Single-atom molybdenum engineered platinum nanocatalyst for boosted alkaline hydrogen oxidation. Adv. Energy Mater. 2022, 12, 2103336.
Zhan, C. H.; Xu, Y.; Bu, L. Z.; Zhu, H. Z.; Feng, Y. G.; Yang, T.; Zhang, Y.; Yang, Z. Q.; Huang, B. L.; Shao, Q. et al. Subnanometer high-entropy alloy nanowires enable remarkable hydrogen oxidation catalysis. Nat. Commun. 2021, 12, 6261.
Hamo, E. R.; Singh, R. K.; Douglin, J. C.; Chen, S. A.; Ben Hassine, M.; Carbo-Argibay, E.; Lu, S. F.; Wang, H. N.; Ferreira, P. J.; Rosen, B. A. et al. Carbide-supported ptru catalysts for hydrogen oxidation reaction in alkaline electrolyte. ACS Catal. 2021, 11, 932–947.
Ni, W. Y.; Meibom, J. L.; Hassan, N. U.; Chang, M.; Chu, Y. C.; Krammer, A.; Sun, S. L.; Zheng, Y. W.; Bai, L. C.; Ma, W. C. et al. Synergistic interactions between ptru catalyst and nitrogen-doped carbon support boost hydrogen oxidation. Nat. Catal. 2023, 6, 773–783.
Wang, S. P.; Fu, L. H.; Huang, H. P.; Fu, M.; Cai, J. L.; Lyu, Z. X.; Wang, Q. X.; Kuang, Q.; Xie, Z. X.; Xie, S. F. Local oxidation induced amorphization of 1.5-nm-thick Pt-Ru nanowires enables superactive and co-tolerant hydrogen oxidation in alkaline media. Adv. Funct. Mater. 2023, 33, 2304125.
Zhou, Y. Y.; Xie, Z. Y.; Jiang, J. X.; Wang, J.; Song, X. Y.; He, Q.; Ding, W.; Wei, Z. D. Lattice-confined Ru clusters with high Co tolerance and activity for the hydrogen oxidation reaction. Nat. Catal. 2020, 3, 454–462.
Wang, Y. H.; Wang, X. T.; Ze, H.; Zhang, X. G.; Radjenovic, P. M.; Zhang, Y. J.; Dong, J. C.; Tian, Z. Q.; Li, J. F. Spectroscopic verification of adsorbed hydroxy intermediates in the bifunctional mechanism of the hydrogen oxidation reaction. Angew. Chem., Int. Ed. 2021, 60, 5708–5711.
Liu, W.; Lyu, K. J.; Xiao, L. T.; Lu, J.; Zhuang, L. Hydrogen oxidation reaction on modified platinum model electrodes in alkaline media. Electrochim. Acta 2019, 327, 135016.
Wang, Y.; Wang, G. W.; Li, G. W.; Huang, B.; Pan, J.; Liu, Q.; Han, J. J.; Xiao, L.; Lu, J. T.; Zhuang, L. Pt-Ru catalyzed hydrogen oxidation in alkaline media: Oxophilic effect or electronic effect. Energy Environ. Sci. 2015, 8, 177–181.
Zhu, S. Q.; Qin, X. P.; Xiao, F.; Yang, S. L.; Xu, Y.; Tan, Z.; Li, J. D.; Yan, J. W.; Chen, Q.; Chen, M. S. et al. The role of ruthenium in improving the kinetics of hydrogen oxidation and evolution reactions of platinum. Nat. Catal. 2021, 4, 711–718.
Mu, X. Q.; Zhang, X. Y.; Chen, Z. Y.; Gao, Y.; Yu, M.; Chen, D.; Pan, H. Z.; Liu, S. L.; Wang, D. S.; Mu, S. C. Constructing symmetry-mismatched RuxFe3−xO4 heterointerface-supported Ru clusters for efficient hydrogen evolution and oxidation reactions. Nano Lett. 2024, 24, 1015–1023.
Zhang, X. Y.; Xiao, X. Z.; Chen, J.; Liu, Y. F.; Pan, H. G.; Sun, W. P.; Gao, M. X. Toward the fast and durable alkaline hydrogen oxidation reaction on ruthenium. Energy Environ. Sci. 2022, 15, 4511–4526.
You, S. H.; Jung, S. M.; Kim, K. S.; Lee, J.; Park, J.; Jang, H. Y.; Shin, S.; Lee, H.; Back, S.; Lee, J. et al. Enhanced durability of automotive fuel cells via selectivity implementation by hydrogen spillover on the electrocatalyst surface. ACS Energy Lett. 2023, 8, 2201–2213.
Li, H.; Wang, X.; Gong, X.; Liu, C.; Ge, J. J.; Song, P.; Xu, W. L. “One stone three birds” of a synergetic effect between Pt single atoms and clusters makes an ideal anode catalyst for fuel cells. J. Mater. Chem. A 2023, 11, 14826–14832.
Xue, Y. R.; Shi, L.; Liu, X. R.; Fang, J. J.; Wang, X. D.; Setzler, B. P.; Zhu, W.; Yan, Y. S.; Zhuang, Z. B. A highly-active, stable and low-cost platinum-free anode catalyst based on RuNi for hydroxide exchange membrane fuel cells. Nat. Commun. 2020, 11, 5651.
Zhang, B. X.; Zhang, B. H.; Zhao, G. Q.; Wang, J. M.; Liu, D. Q.; Chen, Y. P.; Xia, L. X.; Gao, M. X.; Liu, Y. F.; Sun, W. P. et al. Atomically dispersed chromium coordinated with hydroxyl clusters enabling efficient hydrogen oxidation on ruthenium. Nat. Commun. 2022, 13, 5894.
Zhang, X. B.; Xia, L. X.; Zhao, G. Q.; Zhang, B. X.; Chen, Y. P.; Chen, J.; Gao, M. X.; Jiang, Y. Z.; Liu, Y. F.; Pan, H. G. et al. Fast and durable alkaline hydrogen oxidation reaction at the electron-deficient ruthenium–ruthenium oxide interface. Adv. Mater. 2023, 35, 2208821.
Li, Y. B.; Yang, C. Y.; Yue, J. C.; Cong, H. J.; Luo, W. Polymorphism-interface-induced work function regulating on Ru nanocatalyst for enhanced alkaline hydrogen oxidation reaction. Adv. Funct. Mater. 2023, 33, 2211586.
Mahmood, J.; Li, F.; Jung, S. M.; Okyay, M. S.; Ahmad, I.; Kim, S. J.; Park, N.; Jeong, H. Y.; Baek, J. B. An efficient and pH-universal ruthenium-based catalyst for the hydrogen evolution reaction. Nat. Nanotechnol. 2017, 72, 441–446.
Chen, L. G.; Liang, X.; Wang, D. S.; Yang, Z. B.; He, C. T.; Zhao, W.; Pei, J. J.; Xue, Y. R. Platinum-ruthenium single atom alloy as a bifunctional electrocatalyst toward methanol and hydrogen oxidation reactions. ACS Appl. Mater. Interfaces 2022, 14, 27814–27822.
Ding, J. J.; Sun, X. X.; Wang, Q.; Li, D. S.; Li, X. Y.; Li, X. X.; Chen, L.; Zhang, X.; Tian, X. Y.; Ostrikov, K. Plasma synthesis of Pt/g-C3N4 photocatalysts with enhanced photocatalytic hydrogen generation. J. Alloys Compd. 2021, 873, 159871.
Chen, Y. J.; Li, J.; Wang, N.; Zhou, Y. N.; Zheng, J.; Chu, W. Plasma-assisted highly dispersed Pt single atoms on Ru nanoclusters electrocatalyst for pH-universal hydrogen evolution. Chem. Eng. J. 2022, 448, 137611.
Kim, J.; Roh, C. W.; Sahoo, S. K.; Yang, S.; Bae, J.; Han, J. W.; Lee, H. Highly durable platinum single-atom alloy catalyst for electrochemical reactions. Adv. Energy Mater. 2018, 8, 1701476.
Li, M. F.; Duanmu, K. N.; Wan, C. Z.; Cheng, T.; Zhang, L.; Dai, S.; Chen, W. X.; Zhao, Z. P.; Li, P.; Fei, H. L. et al. Single-atom tailoring of platinum nanocatalysts for high-performance multifunctional electrocatalysis. Nat. Catal. 2019, 2, 495–503.
Wang, X. N.; Zhao, L. M.; Li, X. J.; Liu, Y.; Wang, Y. S.; Yao, Q. F.; Xie, J. P.; Xue, Q. Z.; Yan, Z. F.; Yuan, X. et al. Atomic-precision Pt6 nanoclusters for enhanced hydrogen electro-oxidation. Nat. Commun. 2022, 13, 1596.
Kamai, R.; Kamiya, K.; Hashimoto, K.; Nakanishi, S. Oxygen-tolerant electrodes with platinum-loaded covalent triazine frameworks for the hydrogen oxidation reaction. Angew. Chem., Int. Ed. 2016, 55, 13184–13188.
Stühmeier, B. M.; Damjanović, A. M.; Rodewald, K.; Gasteiger, H. A. Selective anode catalyst for the mitigation of start-up/shut-down induced cathode degradation in proton exchange membrane fuel cells. J. Power Sources 2023, 558, 232572.
Men, Y. N.; Wu, D. A.; Hu, Y. C.; Li, L.; Li, P.; Jia, S. F.; Wang, J. B.; Cheng, G. Z.; Chen, S. L.; Luo, W. Understanding alkaline hydrogen oxidation reaction on pdniruirrh high-entropy-alloy by machine learning potential. Angew. Chem., Int. Ed. 2023, 62, e202217976.
Cai, J. L.; Zhang, X.; Lyu, Z. X.; Huang, H. P.; Wang, S. P.; Fu, L. H.; Wang, Q. X.; Yu, X. F.; Xie, Z. X.; Xie, S. F. Host-guest ensemble effect on dual-Pt atom-on-Rh nanosheets enables high-efficiency and anti-CO alkaline hydrogen oxidation. ACS Catal. 2023, 13, 6974–6982.
Gao, L. J.; Wang, Y.; Li, H. B.; Li, Q. H.; Ta, N.; Zhuang, L.; Fu, Q.; Bao, X. H. A nickel nanocatalyst within a h-BN shell for enhanced hydrogen oxidation reactions. Chem. Sci. 2017, 8, 5728–5734.
Cong, Y. Y.; Chai, C. X.; Zhao, X. W.; Yi, B. L.; Song, Y. J. Pt0.25Ru0.75/N-C as highly active and durable electrocatalysts toward alkaline hydrogen oxidation reaction. Adv. Mater. Interfaces 2020, 7, 2000310.
Chen, N. J.; Wang, H. H.; Kim, S. P.; Kim, H. M.; Lee, W. H.; Hu, C.; Bae, J. Y.; Sim, E. S.; Chung, Y. C.; Jang, J. H. et al. Poly (fluorenyl aryl piperidinium) membranes and ionomers for anion exchange membrane fuel cells. Nat. Commun. 2021, 12, 2367.
Dang, Y. L.; Wu, T. L.; Tan, H. Y.; Wang, J. L.; Cui, C.; Kerns, P.; Zhao, W.; Posada, L.; Wen, L. Y.; Suib, S. L. Partially reduced Ru/RuO2 composites as efficient and pH-universal electrocatalysts for hydrogen evolution. Energy Environ. Sci. 2021, 14, 5433–5443.
Cai, C.; Liu, K.; Zhu, Y. M.; Li, P. C.; Wang, Q. Y.; Liu, B.; Chen, S. Y.; Li, H. J. W.; Zhu, L.; Li, H. M. et al. Optimizing hydrogen binding on Ru sites with RuCo alloy nanosheets for efficient alkaline hydrogen evolution. Angew. Chem., Int. Ed. 2022, 61, e202113664.
Wang, J. M.; Zhang, B. X.; Guo, W.; Wang, L.; Chen, J.; Pan, H. G.; Sun, W. P. Toward electrocatalytic methanol oxidation reaction: Longstanding debates and emerging catalysts. Adv. Mater. 2023, 35, 2211099.
Pang, B. B.; Jia, C. Y.; Wang, S. C.; Liu, T.; Ding, T.; Liu, X. K.; Liu, D.; Cao, L. L.; Zhu, M. Z.; Liang, C. H. et al. Self-optimized ligand effect of single-atom modifier in ternary Pt-based alloy for efficient hydrogen oxidation. Nano Lett. 2023, 23, 3826–3834.
Li, F.; Han, G. F.; Jeon, J. P.; Shin, T. J.; Fu, Z. P.; Lu, Y. L.; Baek, J. B. Surface electronic modulation with hetero-single atoms to enhance oxygen evolution catalysis. ACS Nano 2021, 15, 11891–11897.
Chen, C. H.; Wu, D. Y.; Li, Z.; Zhang, R.; Kuai, C. G.; Zhao, X. R.; Dong, C. K.; Qiao, S. Z.; Liu, H.; Du, X. W. Ruthenium-based singleatom alloy with high electrocatalytic activity for hydrogen evolution. Adv. Energy Mater. 2019, 9, 1803913.
Song, X. M.; Zhang, X. G.; Deng, Y. L.; Nan, Z. A.; Song, W. S.; Wang, Y. J.; Lü, L. Z.; Jiang, Q. R.; Jin, X.; Zheng, Y. P. et al. Improving the hydrogen oxidation reaction rate of Ru by active hydrogen in the ultrathin Pd interlayer. J. Am. Chem. Soc. 2023, 145, 12717–12725.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 52171224 and 92261119). J. M. W. acknowledges support from Zhejiang Province Postdoctoral Science Foundation (No. ZJ2022003) and China Postdoctoral Science Foundation (No. 2023M733020). The authors thank the staff of beamline BL11B at the Shanghai Synchrotron Radiation Facility and the staff at Photoemission End-station (BL10B) in the National Synchrotron Radiation Laboratory (NSRL) for their support in XAS measurements.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2024_6604_MOESM1_ESM.pdf
Pt single atoms coupled with Ru nanoclusters enable robust hydrogen oxidation for high-performance anion exchange membrane fuel cells
Rights and permissions
About this article
Cite this article
Wang, J., Zhang, B., Zheng, X. et al. Pt single atoms coupled with Ru nanoclusters enable robust hydrogen oxidation for high-performance anion exchange membrane fuel cells. Nano Res. (2024). https://doi.org/10.1007/s12274-024-6604-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12274-024-6604-7