Abstract
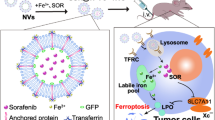
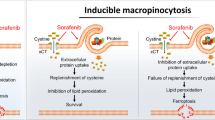
Sorafenib, as a first-line drug for advanced hepatocellular carcinoma (HCC), could trigger ferroptosis by inhibiting cystine/glutamate transporter. However, low-level intracellular iron and insufficient activation of adenosine monophosphate (AMP)-activated protein kinase (AMPK) confer impaired response to sorafenib. In this study, a unique sorafenib nanocomposite dexterously modified with Fe-Material of Institut Lavoisier (sora@Fe-MIL) was synthesized to escalate intracellular iron level and activate AMPK, further potentiating the ferroptotic effect of sorafenib. Remarkably, this strategic deployment of sora@Fe-MIL triggered an extensive demise of cancer cells, while manifesting negligible deleterious impact on normal cells. Two prominent ferroptosis biomarkers, glutathione peroxidase 4 (GPX4) and solute carrier family 7 member 11 (SLC7A11), underwent pronounced downregulation, underscoring the efficacy of this strategy in inducing ferroptosis. Furthermore, the bioactivity of AMPK was considerably elevated, and its downstream targets were conspicuously inhibited by the treatment with sora@Fe-MIL. Using orthotopic HCC animal models, we observed a substantial suppression of primary in situ tumor growth, and ribonucleic acid (RNA) sequencing elucidated an elevated degree of ferroptosis and AMPK activation with the treatment of sora@Fe-MIL. In conclusion, we proposed that the meticulously designed strategy for secure and efficacious iron release and AMPK activation could significantly potentiate the ferroptotic impact of sorafenib, thus resuscitating its therapeutic response in HCC patients.

Similar content being viewed by others
References
Sung, H.; Ferlay, J.; Siegel, R. L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249.
Tang, W. W.; Chen, Z. Y.; Zhang, W. L.; Cheng, Y.; Zhang, B.; Wu, F.; Wang, Q.; Wang, S. J.; Rong, D. W.; Reiter, F. P. et al. The mechanisms of sorafenib resistance in hepatocellular carcinoma: Theoretical basis and therapeutic aspects. Signal Transduct. Target. Ther. 2020, 5, 87.
Depalo, N.; Iacobazzi, R. M.; Valente, G.; Arduino, I.; Villa, S.; Canepa, F.; Laquintana, V.; Fanizza, E.; Striccoli, M.; Cutrignelli, A. et al. Sorafenib delivery nanoplatform based on superparamagnetic iron oxide nanoparticles magnetically targets hepatocellular carcinoma. Nano Res. 2017, 10, 2431–2448.
Louandre, C.; Ezzoukhry, Z.; Godin, C.; Barbare, J. C.; Mazière, J. C.; Chauffert, B.; Galmiche, A. Iron-dependent cell death of hepatocellular carcinoma cells exposed to sorafenib. Int. J. Cancer. 2013, 133, 1732–1742.
Lu, Y. J.; Chan, Y. T.; Tan, H. Y.; Zhang, C.; Guo, W.; Xu, Y.; Sharma, R.; Chen, Z. S.; Zheng, Y. C.; Wang, N. et al. Epigenetic regulation of ferroptosis via ETS1/miR-23a-3p/ACSL4 axis mediates sorafenib resistance in human hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2022, 41, 3.
Gao, R. Z.; Kalathur, R. K. R.; Coto-Llerena, M.; Ercan, C.; Buechel, D.; Shuang, S.; Piscuoglio, S.; Dill, M. T.; Camargo, F. D.; Christofori, G. et al. YAP/TAZ and ATF4 drive resistance to sorafenib in hepatocellular carcinoma by preventing ferroptosis. EMBO Mol. Med. 2021, 13, e14351
Sun, X. F.; Niu, X. H.; Chen, R. C.; He, W. Y.; Chen, D.; Kang, R.; Tang, D. L. Metallothionein-1G facilitates sorafenib resistance through inhibition of ferroptosis. Hepatology 2016, 64, 488–500.
Gao, Z. L.; Wang, D. Y.; Yang, J. X.; Li, M.; Ling, C. Q.; Lv, D. Y.; Cao, Y.; Chen, Z. Y.; Shi, C.; Shen, H. et al. Iron deficiency in hepatocellular carcinoma cells induced sorafenib resistance by upregulating HIF-1a to inhibit apoptosis. Biomed. Pharmacother. 2023, 163, 114750.
Lai, H. Y.; Tsai, H. H.; Yen, C. J.; Hung, L. Y.; Yang, C. C.; Ho, C. H.; Liang, H. Y.; Chen, F. W.; Li, C. F.; Wang, J. M. Metformin resensitizes sorafenib-resistant HCC cells through AMPK-dependent autophagy activation. Front. Cell Dev. Biol. 2021, 8, 596655.
Bort, A.; Sánchez, B. G.; Mateos-Gómez, P. A.; Vara-Ciruelos, D.; Rodríguez-Henche, N.; Díaz-Laviada, I. Targeting AMP-activated kinase impacts hepatocellular cancer stem cells induced by long-term treatment with sorafenib. Mol. Oncol. 2019, 13, 1311–1331.
Li, D. Y.; Yao, Y. C.; Rao, Y. H.; Huang, X. Y.; Wei, L.; You, Z. M.; Zheng, G.; Hou, X. L.; Su, Y.; Varghese, Z. et al. Cholesterol sensor SCAP contributes to sorafenib resistance by regulating autophagy in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2022, 41, 116.
Bi, L.; Ren, Y. D.; Feng, M. X.; Meng, P.; Wang, Q.; Chen, W. P.; Jiao, Q. L.; Wang, Y. L.; Du, L. T.; Zhou, F. Q. et al. HDAC11 regulates glycolysis through the LKB1/AMPK signaling pathway to maintain hepatocellular carcinoma stemness. Cancer Res. 2021, 81, 2015–2028
Ren, Y.; Gu, Y. K.; Li, Z.; Xu, G. Z.; Zhang, Y. M.; Dong, M. X.; Wang, Y.; Zhou, X. B. CXCR3 confers sorafenib resistance of HCC cells through regulating metabolic alteration and AMPK pathway. Am. J. Transl. Res. 2020, 12, 825–836.
Huang, D. D.; Xu, D. F.; Chen, W. X.; Wu, R. M.; Wen, Y. J.; Liu, A. L.; Lin, L. Q.; Lin, X. H.; Wang, X. W. Fe-MnO2 nanosheets loading dihydroartemisinin for ferroptosis and immunotherapy. Biomed. Pharmacother. 2023, 161, 114431.
Chen, X.; Yu, C. H.; Kang, R.; Tang, D. L. Iron metabolism in ferroptosis. Front. Cell Dev. Biol. 2020, 8, 590226.
Yang, Y. L.; Chen, R.; Li, K.; Gao, P. F.; Gong, Y.; Yang, W. H.; Cai, K. Y. Design of manganese ion coated Prussian blue nanocarrier for the therapy of refractory diffuse large B-cell lymphoma based on a comprehensive analysis of ferroptosis regulators from clinical cases. Nano Res. 2023, 16, 5265–5278.
Cheng, J. D.; Huang, T. L.; Li, Y. F.; Guo, Y. B.; Zhu, Y. Z.; Wang, Q. J.; Tan, X. J.; Chen, W. S.; Zhang, Y. N.; Cheng, W. J. et al. AMP-activated protein kinase suppresses the in vitro and in vivo proliferation of hepatocellular carcinoma. PLoS One 2014, 9, e93256.
Ren, C. X.; Hao, X. Q.; Wang, L.; Hu, Y.; Meng, L.; Zheng, S. Z.; Ren, F. L.; Bu, W. H.; Wang, H.; Li, D. W. et al. Metformin carbon dots for promoting periodontal bone regeneration via activation of ERK/AMPK pathway. Adv. Healthc. Mater. 2021, 10, e2100196.
Zheng, Q. Q.; Zhao, Y. S.; Guo, J.; Zhao, S. D.; Fei, C. M.; Xiao, C.; Wu, D.; Wu, L. Y.; Li, X.; Chang, C. K. Iron overload promotes mitochondrial fragmentation in mesenchymal stromal cells from myelodysplastic syndrome patients through activation of the AMPK/MFF/Drp1 pathway. Cell Death Dis. 2018, 9, 515.
Ward, D. M.; Cloonan, S. M. Mitochondrial iron in human health and disease. Annu. Rev. Physiol. 2019, 81, 453–482.
Santambrogio, P.; Dusi, S.; Guaraldo, M.; Rotundo, L. I.; Broccoli, V.; Garavaglia, B.; Tiranti, V.; Levi, S. Mitochondrial iron and energetic dysfunction distinguish fibroblasts and induced neurons from pantothenate kinase-associated neurodegeneration patients. Neurobiol. Dis. 2015, 81, 144–153.
Zhao, Y. B.; Li, M. H.; Yao, X. M.; Fei, Y.; Lin, Z. H.; Li, Z. G.; Cai, K. Y.; Zhao, Y. L.; Luo, Z. HCAR1/MCT1 regulates tumor ferroptosis through the lactate-mediated AMPK-SCD1 activity and its therapeutic implications. Cell Rep. 2020, 33, 108487
Kang, N.; Son, S.; Min, S. H.; Hong, H.; Kim, C.; An, J.; Kim, J. S.; Kang, H. Stimuli-responsive ferroptosis for cancer therapy. Chem. Soc. Rev. 2023, 52, 3955–3972.
Liu, K. X.; Huang, L.; Qi, S. Y.; Liu, S. C.; Xie, W. N.; Du, L. Y.; Cui, J.; Zhang, X.; Zhang, B. Y.; Liu, L. J. et al. Ferroptosis: The entanglement between traditional drugs and nanodrugs in tumor therapy. Adv. Healthc. Mater. 2023, 12, 2203085.
Liu, S. W.; Zhang, M. S.; Jin, H.; Wang, Z.; Liu, Y.; Zhang, S. L.; Zhang, H. Iron-containing protein-mimic supramolecular iron delivery systems for ferroptosis tumor therapy. J. Am. Chem. Soc. 2023, 145, 160–170.
Xu, M.; Zha, H. D.; Han, R.; Cheng, Y. X.; Chen, J. M.; Yue, L. D.; Wang, R. B.; Zheng, Y. Cyclodextrin-derived ROS-generating nanomedicine with pH-modulated degradability to enhance tumor ferroptosis therapy and chemotherapy. Small 2022, 18, 2200330.
Mao, S. H.; Li, S. Y.; Zhang, Y. X.; Long, L. X.; Peng, J. F.; Cao, Y. Y.; Mao, J. Z.; Qi, X.; Xin, Q.; San, G. L. et al. A highly efficient needle-free-injection delivery system for mRNA-LNP vaccination against SARS-CoV-2. Nano Today 2023, 48, 101730.
Wu, C. Y.; Xu, D. L.; Ge, M.; Luo, J. J.; Chen, L. S.; Chen, Z. X.; You, Y. L.; Zhu, Y. X.; Lin, H.; Shi, J. L. Blocking glutathione regeneration: Inorganic NADPH oxidase nanozyme catalyst potentiates tumoral ferroptosis. Nano Today 2022, 46, 101574.
Gordon, J.; Kazemian, H.; Rohani, S. MIL-53(Fe), MIL-101, and SBA-15 porous materials: Potential platforms for drug delivery. Mater. Sci. Eng.: C 2015, 47, 172–179.
Yang, J.; Yang, Y. W. Metal–organic frameworks for biomedical applications. Small 2020, 16, 1906846.
Pham, M. H.; Vuong, G. T.; Vu, A. T.; Do, T. O. Novel route to size-controlled Fe–MIL-88B–NH2 metal–organic framework nanocrystals. Langmuir 2011, 27, 15261–15267.
Yang, B. W.; Ding, L.; Yao, H. L.; Chen, Y.; Shi, J. L. A metal–organic framework (MOF) Fenton nanoagent-enabled nanocatalytic cancer therapy in synergy with autophagy inhibition. Adv. Mater. 2020, 32, 1907152.
Paşcalău, V.; Tertis, M.; Pall, E.; Suciu, M.; Marinca, T.; Pustan, M.; Merie, V.; Rus, I.; Moldovan, C.; Topala, T. et al. Bovine serum albumin gel/polyelectrolyte complex of hyaluronic acid and chitosan based microcarriers for sorafenib targeted delivery. J. Appl. Polym. Sci. 2020, 137, 49002.
Diao, Y. X.; Liu, H. M.; Yao, Z. X.; Liu, Y. S.; Hu, G. X.; Zhang, Q. F.; Li, Z. Tri-(Fe/F/N)-doped porous carbons as electrocatalysts for the oxygen reduction reaction in both alkaline and acidic media. Nanoscale 2020, 12, 18826–18833.
Xue, C. C.; Li, M. H.; Liu, C. H.; Li, Y. N.; Fei, Y.; Hu, Y.; Cai, K. Y.; Zhao, Y. L.; Luo, Z. NIR-actuated remote activation of ferroptosis in target tumor cells through a photothermally responsive iron-chelated biopolymer nanoplatform. Angew. Chem., Int. Ed. 2021, 60, 8938–8947.
Du, J. H.; Zhou, M. T.; Chen, Q.; Tao, Y. C.; Ren, J.; Zhang, Y.; Qin, H. L. Disrupting intracellular iron homeostasis by engineered metal–organic framework for nanocatalytic tumor therapy in synergy with autophagy amplification-promoted ferroptosis. Adv. Funct. Mater. 2023, 33, 2215244.
Liang, W. B.; Wied, P.; Carraro, F.; Sumby, C. J.; Nidetzky, B.; Tsung, C. K.; Falcaro, P.; Doonan, C. J. Metal–organic framework-based enzyme biocomposites. Chem. Rev. 2021, 121, 1077–1129.
Suresh, K.; Matzger, A. J. Enhanced drug delivery by dissolution of amorphous drug encapsulated in a water unstable metal–organic framework (MOF). Angew. Chem., Int. Ed. 2019, 58, 16790–16794.
Wu, M. X.; Yang, Y. W. Metal–organic framework (MOF)-based drug/cargo delivery and cancer therapy. Adv. Mater. 2017, 29, 1606134.
Yang, W. S.; Stockwell, B. R. Ferroptosis: Death by lipid peroxidation. Trends Cell Biol. 2016, 26, 165–176.
Wu, C. Y.; Liu, Z. L.; Chen, Z. X.; Xu, D. L.; Chen, L. S.; Lin, H.; Shi, J. L. A nonferrous ferroptosis-like strategy for antioxidant inhibition-synergized nanocatalytic tumor therapeutics. Sci. Adv. 2021, 7, eabj8833.
Zhang, J. Y.; Qin, Y.; Wang, Z. C.; Zhang, W.; Liu, S. J.; Wei, W.; Wang, X. X.; Zhao, J. Ferroptosis-inducing inorganic arsenic(II) sulfide nanocrystals enhance immune activation. Nano Res. 2023, 16, 9760–9767.
Yang, W. S.; SriRamaratnam, R.; Welsch, M. E.; Shimada, K.; Skouta, R.; Viswanathan, V. S.; Cheah, J. H.; Clemons, P. A.; Shamji, A. F.; Clish, C. B. et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014, 156, 317–331.
Stockwell, B. R.; Friedmann Angeli, J. P.; Bayir, H.; Bush, A. I.; Conrad, M.; Dixon, S. J.; Fulda, S.; Gascón, S.; Hatzios, S. K.; Kagan, V. E. et al. Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell 2017, 171, 273–285.
Zhang, L.; Huang, Y.; Ling, J. J.; Zhuo, W. L.; Yu, Z.; Luo, Y. B.; Zhu, Y. Overexpression of SLC7A11: A novel oncogene and an indicator of unfavorable prognosis for liver carcinoma. Future Oncol. 2018, 14, 927–936.
Koppula, P.; Zhang, Y. L.; Zhuang, L.; Gan, B. Y. Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun. 2018, 38, 12.
Koppula, P.; Zhuang, L.; Gan, B. Y. Cystine transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 2021, 12, 599–620.
Zhang, L.; Li, X. M.; Shi, X. H.; Ye, K.; Fu, X. L.; Wang, X.; Guo, S. M.; Ma, J. Q.; Xu, F. F.; Sun, H. M. et al. Sorafenib triggers ferroptosis via inhibition of HBXIP/SCD axis in hepatocellular carcinoma. Acta Pharmacol. Sin. 2023, 44, 622–634.
Ma, S.; Henson, E. S.; Chen, Y.; Gibson, S. B. Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells. Cell Death Dis. 2016, 7, e2307.
Li, K.; Xu, K.; He, Y.; Lu, L.; Mao, Y. L.; Gao, P. F.; Liu, G. H.; Wu, J.; Zhang, Y. C.; Xiang, Y. et al. Functionalized tumor-targeting nanosheets exhibiting Fe(II) overloading and GSH consumption for ferroptosis activation in liver tumor. Small 2021, 17, 2102046.
Sun, X. F.; Ou, Z. H.; Chen, R. C.; Niu, X. H.; Chen, D.; Kang, R.; Tang, D. L. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology 2016, 63, 173–184.
Dixon, S. J.; Lemberg, K. M.; Lamprecht, M. R.; Skouta, R.; Zaitsev, E. M.; Gleason, C. E.; Patel, D. N.; Bauer, A. J.; Cantley, A. M.; Yang, W. S. et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072.
Jian, C. S.; Fu, J. J.; Cheng, X.; Shen, L. J.; Ji, Y. X.; Wang, X. M.; Pan, S.; Tian, H.; Tian, S.; Liao, R. F. et al. Low-dose sorafenib acts as a mitochondrial uncoupler and ameliorates nonalcoholic steatohepatitis. Cell Metab. 2020, 31, 892–908.e11.
Wang, K.; Zhang, Z. Y.; Tsai, H. I.; Liu, Y. F.; Gao, J.; Wang, M.; Song, L.; Cao, X. F.; Xu, Z. X.; Chen, H. B. et al. Branched-chain amino acid aminotransferase 2 regulates ferroptotic cell death in cancer cells. Cell Death Differ. 2021, 28, 1222–1236.
Mehta, K. J. Role of iron and iron-related proteins in mesenchymal stem cells: Cellular and clinical aspects. J. Cell. Physiol. 2021, 236, 7266–7289.
Faubert, B.; Boily, G.; Izreig, S.; Griss, T.; Samborska, B.; Dong, Z. F.; Dupuy, F.; Chambers, C.; Fuerth, B. J.; Viollet, B. et al. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab. 2013, 17, 113–124.
Das, U. N. Saturated fatty acids, MUFAs and PUFAs regulate ferroptosis. Cell Chem. Biol. 2019, 26, 309–311.
Tesfay, L.; Paul, B. T.; Konstorum, A.; Deng, Z. Y.; Cox, A. O.; Lee, J.; Furdui, C. M.; Hegde, P.; Torti, F. M.; Torti, S. V. Stearoyl-CoA desaturase 1 protects ovarian cancer cells from ferroptotic cell death. Cancer Res. 2019, 79, 5355–5366.
Zhu, X. P.; Bian, H.; Wang, L.; Sun, X. Y.; Xu, X.; Yan, H. M.; Xia, M. F.; Chang, X. X.; Lu, Y.; Li, Y. et al. Berberine attenuates nonalcoholic hepatic steatosis through the AMPK-SREBP-1c-SCD1 pathway. Free Radical Biol. Med. 2019, 141, 192–204.
Schindler, M.; Pendzialek, M.; Grybel, K. J.; Seeling, T.; Gürke, J.; Fischer, B.; Navarrete Santos, A. Adiponectin stimulates lipid metabolism via AMPK in rabbit blastocysts. Hum. Reprod. 2017, 32, 1382–1392.
Lingesh, A.; Paul, D.; Naidu, V.; Satheeshkumar, N. AMPK activating and anti adipogenic potential of Hibiscus rosa sinensis flower in 3T3-L1 cells. J. Ethnopharmacol. 2019, 233, 123–130.
Fang, K.; Wu, F.; Chen, G.; Dong, H.; Li, J. B.; Zhao, Y.; Xu, L. J.; Zou, X.; Lu, F. E. Diosgenin ameliorates palmitic acid-induced lipid accumulation via AMPK/ACC/CPT-1A and SREBP-1c/FAS signaling pathways in LO2 cells. BMC Complement. Altern. Med. 2019, 19, 255.
Xu, H. Y.; Lyu, X.; Guo, X. N.; Yang, H. B.; Duan, L.; Zhu, H. J.; Pan, H.; Gong, F. Y.; Wang, L. J. Distinct AMPK-mediated FAS/HSL pathway is implicated in the alleviating effect of nuciferine on obesity and hepatic steatosis in HFD-fed mice. Nutrients 2022, 14, 1898.
Cui, Y.; Zhang, Y.; Zhao, X. L.; Shao, L. M.; Liu, G. P.; Sun, C. J.; Xu, R.; Zhang, Z. L. ACSL4 exacerbates ischemic stroke by promoting ferroptosis-induced brain injury and neuroinflammation. Brain Behav. Immun. 2021, 93, 312–321
Cheng, L.; Zhu, X. D.; Liu, Y.; Zhu, K.; Lin, K.; Li, F. J. ACSL4 contributes to sevoflurane-induced ferroptotic neuronal death in SH-SY5Y cells via the 5′ AMP-activated protein kinase/mammalian target of rapamycin pathway. Ann. Transl. Med. 2021, 9, 1454.
Milman, T.; Zhang, Q.; Ang, S. M.; Elder, D.; Lally, S. E.; Shields, J. A.; Hamershock, R. A.; Sioufi, K.; Shields, C. L.; Eagle, R. C. Jr. Immunohistochemical profiling of conjunctival melanocytic intraepithelial lesions, including SOX10, HMB45, Ki67, and P16. Am. J. Ophthalmol. 2021, 222, 148–156.
Wang, Y.; Wei, Z. H.; Pan, K. R.; Li, J.; Chen, Q. M. The function and mechanism of ferroptosis in cancer. Apoptosis 2020, 25, 786–798.
Wang, L.; Ge, X. W.; Zhang, Z. F.; Ye, Y. T.; Zhou, Z. Y.; Li, M. H.; Yan, H. X.; Wu, L.; Bai, Q.; Li, J. P. et al. Identification of a ferroptosis-related long noncoding RNA prognostic signature and its predictive ability to immunotherapy in hepatocellular carcinoma. Front. Genet. 2021, 12, 682082.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Nos. 82173143 and 82373409). The authors also acknowledge the support from the Top Young Talents Project of the Special Support Program for High-Level Talents in Shaanxi Province (2020–2025), the Fundamental Research Funds for the Central Universities (Nos. D5000210635 and D5000210829), and General Key R&D Projects in Shaanxi Province (No. 2024SF-YBXM-439).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zhu, J., Zhao, S., Zhu, Y. et al. Sorafenib sensitization in tumor therapy by iron overload and AMPK activation. Nano Res. (2024). https://doi.org/10.1007/s12274-024-6602-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12274-024-6602-9