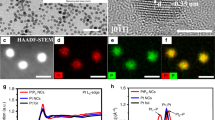
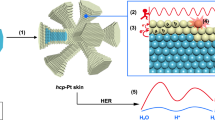
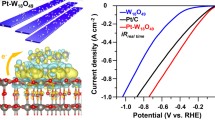
Abstract
The intermittent nature of renewable energy sources sets a requirement for efficient energy storage to mitigate the conflict between energy supply and demand. Hydrogen is a promising choice for energy storage due to its high energy density. However, the conversion of electrical energy to chemical energy stored in hydrogen through water electrolysis suffers from low efficiency, and the electricity cost dominates the total cost of hydrogen production. Here, we report the study of improving the hydrogen evolution reaction activity of Pt-based catalysts by building a nanoscale surface NiO and Pt interface, further optimizing the performance via tuning the lattice parameter of the core of nanoparticles, which can be achieved by varying the dealloying annealing time. The optimized PtCuNi-O/C and PtNi-O/C catalysts are demonstrated to be one of the best catalysts, with a mass activity (MA) of 9.1 and 8.7 mA/µgPt, which is 9.9-fold and 9.5-fold of that of Pt/C, respectively.

Similar content being viewed by others
References
Bauer, N.; Mouratiadou, I.; Luderer, G.; Baumstark, L.; Brecha, R. J.; Edenhofer, O.; Kriegler, E. Global fossil energy markets and climate change mitigation-an analysis with REMIND. Climatic Change 2016, 136, 69–82.
Moriarty, P.; Honnery, D. Can renewable energy power the future. Energy Policy 2016, 93, 3–7.
Dunn, B.; Kamath, H.; Tarascon, J. M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935.
Kalinci, Y.; Hepbasli, A.; Dincer, I. Techno-economic analysis of a stand-alone hybrid renewable energy system with hydrogen production and storage options. Int. J. Hydrogen Energy 2015, 40, 7652–7664.
Katsounaros, I.; Cherevko, S. R., Zeradjanin, A. R.; Mayrhofer, K. J. J. Oxygen electrochemistry as a cornerstone for sustainable energy conversion. Angew. Chem., Int. Ed. 2014, 53, 102–121.
Pellow, M. A.; Emmott, C. J. M.; Barnhart, C. J.; Benson, S. M. Hydrogen or batteries for grid storage? A net energy analysis. Energy Environ. Sci. 2015, 8, 1938–1952.
Pan, F. H. K.; Jin, T.; Yang, W. W.; Li, H.; Cao, Y. Q.; Hu, J.; Zhou, X. G.; Liu, H. L.; Duan, X. Z. Theory-guided design of atomic Fe-Ni dual sites in N,P-co-doped C for boosting oxygen evolution reaction. Chem Catal. 2021, 1, 734–745.
Anantharaj, S.; Kundu, S.; Noda, S. “The Fe effect”: A review unveiling the critical roles of Fe in enhancing OER activity of Ni and Co based catalysts. Nano Energy 2021, 80, 105514.
Seh, Z. W.; Kibsgaard, J.; Dickens, C. F.; Chorkendorff, I.; Nørskov, J. K.; Jaramillo, T. F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998.
Sheng, W. C.; Gasteiger, H. A.; Shao-Horn, Y. Hydrogen oxidation and evolution reaction kinetics on platinum: Acid vs alkaline electrolytes. J. Electrochem. Soc. 2010, 157, B1529.
Skúlason, E.; Tripkovic, V.; Björketun, M. E.; Gudmundsdóttir, S.; Karlberg, G.; Rossmeisl, J.; Bligaard, T.; Jónsson, H.; Nørskov, J. K. Modeling the electrochemical hydrogen oxidation and evolution reactions on the basis of density functional theory calculations. J. Phys. Chem. C 2010, 114, 18182–18197.
Sverdrup, H. U.; Ragnarsdottir, K. V. A system dynamics model for platinum group metal supply, market price, depletion of extractable amounts, ore grade, recycling and stocks-in-use. Resour. Conserv. Recy. 2016, 114, 130–152.
Chen, J. M. Carbon neutrality: Toward a sustainable future. Innovation 2021, 2, 100127.
Chen, Y.; Lai, Z. C.; Zhang, X.; Fan, Z. X.; He, Q. Y.; Tan, C. L.; Zhang, H. Phase engineering of nanomaterials. Nat. Rev. Chem. 2020, 4, 243–256.
Wang, X. G.; Kolen’ko, Y. V.; Bao, X. Q.; Kovnir, K.; Liu, L. F. One-step synthesis of self-supported nickel phosphide nanosheet array cathodes for efficient electrocatalytic hydrogen generation. Angew. Chem., Int. Ed. 2015, 54, 8188–8192.
Tan, Y. W.; Wang, H.; Liu, P.; Cheng, C.; Zhu, F.; Hirata, A.; Chen, M. W. 3D nanoporous metal phosphides toward high-efficiency electrochemical hydrogen production. Adv. Mater. 2016, 28, 2951–2955.
Jaramillo, T. F.; Jørgensen, K. P.; Bonde, J.; Nielsen, J. H.; Horch, S.; Chorkendorff, I. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 2007, 317, 100–102.
Zhang, L.; Xiong, K.; Chen, S. G.; Li, L.; Deng, Z. H.; Wei, Z. D. In situ growth of ruthenium oxide-nickel oxide nanorod arrays on nickel foam as a binder-free integrated cathode for hydrogen evolution. J. Power Sources 2015, 274, 114–120.
Gong, M.; Zhou, W.; Tsai, M. C.; Zhou, J. G.; Guan, M. Y.; Lin, M. C.; Zhang, B.; Hu, Y. F.; Wang, D. Y.; Yang, J. et al. Nanoscale nickel oxide/nickel heterostructures for active hydrogen evolution electrocatalysis. Nat. Commun. 2014, 5, 4695.
Jakšić, J. M.; Vojnović, M. V.; Krstajić, N. V. Kinetic analysis of hydrogen evolution at Ni-Mo alloy electrodes. Electrochim. Acta 2000, 45, 4151–4158.
Raj, I. A.; Vasu, K. I. Transition metal-based hydrogen electrodes in alkaline solution—electrocatalysis on nickel based binary alloy coatings. J. Appl. Electrochem. 1990, 20, 32–38.
Xu, J.; Cui, J. B.; Guo, C.; Zhao, Z. P.; Jiang, R.; Xu, S. Y.; Zhuang, Z. B.; Huang, Y.; Wang, L. Y.; Li, Y. D. Ultrasmall Cu7S4@MoS2 hetero-nanoframes with abundant active edge sites for ultrahigh-performance hydrogen evolution. Angew. Chem., Int. Ed. 2016, 55, 6502–6505.
Ahn, H. S.; Bard, A. J. Assessment of the stability and operability of cobalt phosphide electrocatalyst for hydrogen evolution. Anal. Chem. 2017, 89, 8574–8579.
Cao, Z. M.; Chen, Q. L.; Zhang, J. W.; Li, H. Q.; Jiang, Y. Q.; Shen, S. Y.; Fu, G.; Lu, B. A.; Xie, Z. X.; Zheng, L. S. Platinum-nickel alloy excavated nano-multipods with hexagonal close-packed structure and superior activity towards hydrogen evolution reaction. Nat. Commun. 2017, 8, 15131.
Wang, P. T.; Zhang, X.; Zhang, J.; Wan, S.; Guo, S. J.; Lu, G.; Yao, J. L.; Huang, X. Q. Precise tuning in platinum-nickel/nickel sulfide interface nanowires for synergistic hydrogen evolution catalysis. Nat. Commun. 2017, 8, 14580.
Chen, C.; Kang, Y. J.; Huo, Z. Y.; Zhu, Z. W.; Huang, W. Y.; Xin, H. L.; Snyder, J. D.; Li, D. G.; Herron, J. A.; Mavrikakis, M. et al. Highly crystalline multimetallic nanoframes with three-dimensional electrocatalytic surfaces. Science 2014, 343, 1339–1343.
Oh, A.; Sa, Y. J.; Hwang, H.; Baik, H.; Kim, J.; Kim, B.; Joo, S. H.; Lee, K. Rational design of Pt-Ni-Co ternary alloy nanoframe crystals as highly efficient catalysts toward the alkaline hydrogen evolution reaction. Nanoscale 2016, 8, 16379–16386.
Subbaraman, R.; Tripkovic, D.; Strmcnik, D.; Chang, K. C.; Uchimura, M.; Paulikas, A. P.; Stamenkovic, V.; Markovic, N. M. Enhancing hydrogen evolution activity in water splitting by tailoring Li+Ni(OH)2-Pt Interfaces. Science 2011, 334, 1256–1260.
Yin, H. J.; Zhao, S. L.; Zhao, K.; Muqsit, A.; Tang, H. J.; Chang, L.; Zhao, H. J.; Gao, Y.; Tang, Z. Y. Ultrathin platinum nanowires grown on single-layered nickel hydroxide with high hydrogen evolution activity. Nat. Commun. 2015, 6, 6430.
Wang, P. T.; Jiang, K. Z.; Wang, G. M.; Yao, J. L.; Huang, X. Q. Phase and interface engineering of platinum-nickel nanowires for efficient electrochemical hydrogen evolution. Angew. Chem., Int. Ed. 2016, 55, 12859–12863.
Wang, L.; Zhu, Y. H.; Zeng, Z. H.; Lin, C.; Giroux, M.; Jiang, L.; Han, Y.; Greeley, J.; Wang, C.; Jin, J. Platinum-nickel hydroxide nanocomposites for electrocatalytic reduction of water. Nano Energy 2017, 31, 456–461.
Ma, M. Y.; Cheng, X.; Shi, Z. D.; Zhang, C. L.; Li, Y.; Yang, Y. F.; Gong, C. S.; Zhang, Z. X.; Fei, H. L.; Zhu, C. et al. Role of N in transition-metal-nitrides for anchoring platinum-group metal atoms toward single-atom catalysis. Small Methods 2022, 6, 2200295.
Han, X. P.; Ling, X. F.; Yu, D. S.; Xie, D. Y.; Li, L. L.; Peng, S. J.; Zhong, C.; Zhao, N. Q.; Deng, Y. D.; Hu, W. B. Atomically dispersed binary Co-Ni sites in nitrogen-doped hollow carbon nanocubes for reversible oxygen reduction and evolution. Adv. Mater. 2019, 31, 1905622.
Park, E. J.; Arges, C. G.; Xu, H.; Kim, Y. S. Membrane strategies for water electrolysis. ACS Energy Lett. 2022, 7, 3447–3457.
Sun, Q.; Oliveira, N. J.; Kwon, S.; Tyukhtenko, S.; Guo, J. J.; Myrthil, N.; Lopez, S. A.; Kendrick, I.; Mukerjee, S.; Ma, L. et al. Understanding hydrogen electrocatalysis by probing the hydrogen-bond network of water at the electrified Pt-solution interface. Nat. Energy 2023, 8, 859–869.
Liu, C.; Zhang, P. F.; Liu, B.; Meng, Q.; Yang, X. Z.; Li, Y. K.; Han, J. L.; Wang, Y. Long-range Pt-Ni dual sites boost hydrogen evolution through optimizing the adsorption configuration. Nano Res. 2024, 17, 3700–3706.
Wang, Y.; Zheng, M.; Li, Y. R.; Chen, J.; Ye, J. Y.; Ye, C. L.; Li, S. N.; Wang, J.; Zhu, Y. F.; Sun, S. G. et al. Oxygen-bridged long-range dual sites boost ethanol electrooxidation by facilitating C-C bond cleavage. Nano Lett. 2023, 23, 8194–8202.
Wang, Y.; Zheng, M.; Li, Y. R.; Ye, C. L.; Chen, J.; Ye, J. Y.; Zhang, Q. H.; Li, J.; Zhou, Z. Y.; Fu, X. Z. et al. p-d orbital hybridization induced by a monodispersed Ga site on a Pt3Mn nanocatalyst boosts ethanol electrooxidation. Angew. Chem., Int. Ed. 2022, 61, e202115735.
Lin, L.; Sun, Z. M.; Yao, H. Y.; Yuan, M. W.; Yang, H.; Li, H. F.; Zhang, Q. H.; Wang, D. W.; Gu, L.; Sun, G. B. et al. Tuning surface lattice strain toward a Pt-Skin CoPtx truncated octahedron for hydrogen evolution reaction. J. Phys. Chem. C 2019, 123, 29722–29728.
He, T. O.; Wang, W. C.; Shi, F. L.; Yang, X. L.; Li, X.; Wu, J. B.; Yin, Y. D.; Jin, M. S. Mastering the surface strain of platinum catalysts for efficient electrocatalysis. Nature 2021, 598, 76–81.
Zhao, Z. P.; Liu, H. T.; Gao, W. P.; Xue, W.; Liu, Z. Y.; Huang, J.; Pan, X. Q.; Huang, Y. Surface-engineered PtNi-O nanostructure with record-high performance for electrocatalytic hydrogen evolution reaction. J. Am. Chem. Soc. 2018, 140, 9046–9050.
Cao, L.; Zhao, Z. P.; Liu, Z. Y.; Gao, W. P.; Dai, S.; Gha, J.; Xue, W.; Sun, H. T.; Duan, X. F.; Pan, X. Q. et al. Differential surface elemental distribution leads to significantly enhanced stability of PtNi-based ORR catalysts. Matter 2019, 1, 1567–1580.
Zhao, Z. P.; Feng, M.; Zhou, J. H.; Liu, Z. Y.; Li, M. F.; Fan, Z.; Tsen, O.; Miao, J. W.; Duan, X. F.; Huang, Y. Composition tunable ternary Pt-Ni-Co octahedra for optimized oxygen reduction activity. Chem. Commun. 2016, 52, 11215–11218.
Huang, X. Q.; Zhao, Z. P.; Chen, Y.; Zhu, E. B.; Li, M. F.; Duan, X. F.; Huang, Y. A rational design of carbon-supported dispersive Pt-based octahedra as efficient oxygen reduction reaction catalysts. Energy Environ. Sci. 2014, 7, 2957–2962.
Yin, Y. D.; Rioux, R. M.; Erdonmez, C. K.; Hughes, S.; Somorjai, G. A.; Alivisatos, A. P. Formation of hollow nanocrystals through the nanoscale kirkendall effect. Science 2004, 304, 711–714.
Zhu, Y. B.; Liu, T.; Li, L. M.; Song, S. L.; Ding, R. Nickel-based electrodes as catalysts for hydrogen evolution reaction in alkaline media. Ionics 2018, 24, 1121–1127.
Shinagawa, T.; Garcia-Esparza, A. T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801.
Acknowledgements
Y. H., X. F. D., Q. Y. J., Z. P. Z., H. T. L., and Z. Y. L. acknowledge support from the Office of Naval Research by the grant number N000141812155 (Program officer Laura Kienker, laura.kienker@navy.mil). The imaging work at UC Irvine is supported by the National Science Foundation with grant numbers CBET 1159240, DMR-1420620, and DMR-1506535. Use of Beamline 7-BM (QAS) of the National Synchrotron Light Source (NSLS) II was supported by the NSLS-II, Brookhaven National Laboratory, under U.S. DOE Contract No. DE-SC0012704. J. H. G. at the Advanced Light Source is supported by the DOE Office of Science under contract No. DE-AC02-05CH11231. We thank the support from Irvine Materials Research Institute (IMRI) at the University of California Irvine for TEM work conducted on JEOL Grand ARM. We thank the help from Dr. Shang-Hsien Hsieh for collecting some EXAFS data.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Zhao, Z., Liu, H., Gao, W. et al. Improving hydrogen evolution reaction efficiency through lattice tuning. Nano Res. (2024). https://doi.org/10.1007/s12274-024-6579-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12274-024-6579-4