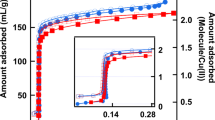
Abstract
Porous heterogeneous lyophobic systems (HLSs) find potential applications in energy restoring, dissipating, and absorbing. However, the development of controllable HLSs still lacks rational structure design of nanoporous materials matching the molecular sizes of adopted liquids. Besides that, thoroughly understanding the underlying transportation mechanism in the confined nano channels is greatly challenging. In this work, a series of Co/Zn bimetallic zeolitic imidazolate frameworks (ZIFs) with tunable structures were synthesized via regulating the Co to Zn ratios and employed to investigate the intrusion–extrusion of liquid water in confined nanopores. Structural characterizations confirm the heterometallic coordination in the Co/Zn-doped frameworks. Water intrusion–extrusion experiments unlock the relationship between the intrusion pressure and the nanopore size and realize the evolution of the HLSs between molecular spring and shock-absorber. In addition, cycling tests indicate the reversible structure change of Co/Zn ZIFs encountering pressure-induced water intrusion. In combination with molecular dynamics simulations, we present that the water multimers intrude into nanopores of ZIFs in chain-like forms along with dissociation of hydrogen bonds (HBs). Water molecules in the pre-intrusion state exhibit reduced HBs in response to the increase of pressure and linear structure with 1.6–3.0 HBs on average. After transition to the post-intrusion situation, the associative configuration of water tends to exhibit the tetrahedral structure. Herein, we highlight the roles of pore size and HB in synergically dominating the pressure-induced intrusion–extrusion of liquid water in hydrophobic nanopores. Furthermore, the present work can also guide the development of functional guest–host systems based on porous architectures.

Similar content being viewed by others
References
Goeminne, R.; Krause, S.; Kaskel, S.; Verstraelen, T.; Evans, J. D. Charting the complete thermodynamic landscape of gas adsorption for a responsive metal-organic framework. J. Am. Chem. Soc. 2021, 143, 4143–4147.
Yang, Y. B.; Yang, X. D.; Liang, L.; Gao, Y. Y.; Cheng, H. Y.; Li, X. M.; Zou, M. C.; Ma, R. Z.; Yuan, Q. Duan, X. F. Large-area graphene-nanomesh/carbon-nanotube hybrid membranes for ionic and molecular nanofiltration. Science 2019, 364, 1057–1062.
Fang, W.; Wang, C. T.; Liu, Z. Q.; Wang, L.; Liu, L.; Li, H. J.; Xu, S. D.; Zheng, A. M.; Qin, X. D.; Liu, L. J. et al. Physical mixing of a catalyst and a hydrophobic polymer promotes CO hydrogenation through dehydration. Science 2022, 377, 406–410.
Zhang, W. Y.; Wei, S.; Wu, Y. N.; Wang, Y. L.; Zhang, M.; Roy, D.; Wang, H.; Yuan, J. Y.; Zhao, Q. Poly(ionic liquid)-derived graphitic nanoporous carbon membrane enables superior supercapacitive energy storage. ACS Nano 2019, 13, 10261–10271.
Lee, A.; Hudson, A. R.; Shiwarski, D. J.; Tashman, J. W.; Hinton, T. J.; Yerneni, S.; Bliley, J. M.; Campbell, P. G.; Feinberg, A. W. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487.
El-Kady, M. F.; Strong, V.; Dubin, S.; Kaner, R. B. Laser scribing of high-performance and flexible graphene-based electrochemical capacitors. Science 2012, 335, 1326–1330.
Eroshenko, V.; Regis, R. C.; Soulard, M.; Patarin, J. Energetics: A new field of applications for hydrophobic zeolites. J. Am. Chem. Soc. 2001, 123, 8129–8130.
Yu, M. C.; Chen, Q.; Gao, X. Theoretical and experimental investigation of molecular spring isolator. Microsyst. Technol. 2017, 23, 285–292.
Zhou, X.; Miao, Y. R.; Shaw, W. L.; Suslick, K. S.; Dlott, D. D. Shock wave energy absorption in metal-organic framework. J. Am. Chem. Soc. 2019, 141, 2220–2223.
Tinti, A.; Giacomello, A.; Grosu, Y.; Casciola, C. M. Intrusion and extrusion of water in hydrophobic nanopores. Proc. Natl. Acad. Sci. USA 2017, 114, E10266–E10273.
Fraux, G.; Coudert, F. X.; Boutin, A.; Fuchs, A. H. Forced intrusion of water and aqueous solutions in microporous materials: From fundamental thermodynamics to energy storage devices. Chem. Soc. Rev. 2017, 46, 7421–7437.
Liu, L.; Chen, X.; Lu, W. Y.; Han, A. J.; Qiao, Y. Infiltration of electrolytes in molecular-sized nanopores. Phys. Rev. Lett. 2009, 102, 184501.
Zhao, J. B.; Culligan, P. J.; Germaine, J. T.; Chen, X. Experimental study on energy dissipation of electrolytes in nanopores. Langmuir 2009, 25, 12687–12696.
Pillot, M.; Lebeau, B.; Nouali, H.; Daou, T. J.; Patarin, J.; Ryzhikov, A. High pressure intrusion of water and LiCl aqueous solutions in hydrophobic KIT-6 mesoporous silica: Influence of the grafted group nature. Micropor. Mesopor. Mater. 2019, 280, 248–255.
Huve, J.; Daou, T. J.; Nouali, H.; Patarin, J.; Ryzhikov, A. The effect of nanostructures on high pressure intrusion-extrusion of water and electrolyte solutions in hierarchical nanoboxes of silicalite-1. New J. Chem. 2020, 44, 273–281.
Isaac, C.; Confalonieri, G.; Nouali, H.; Paillaud, J. L.; Arletti, R.; Daou, T. J.; Ryzhikov, A. Unusual high-pressure intrusion-extrusion behavior of electrolyte solutions in Mu-26, a pure silica zeolite of topology STF. Micropor. Mesopor. Mater. 2020, 298, 110047.
Trzpit, M.; Soulard, M.; Patarin, J. The pure silica Chabazite: A high volume molecular spring at low pressure for energy storage. Chem. Lett. 2007, 36, 980–981.
Qiao, Y.; Punyamurtula, V. K.; Xian, G. J.; Karbhari, V. M.; Han, A. J. Conversion of mechanical work to interfacial tension in a nanoporous silica gel. Appl. Phys. Lett. 2008, 92, 063109.
Chen, X.; Surani, F. B.; Kong, X. G.; Punyamurtula, V. K.; Qiao, Y. Energy absorption performance of steel tubes enhanced by a nanoporous material functionalized liquid. Appl. Phys. Lett. 2006, 89, 241918.
Trzpit, M.; Rigolet, S.; Paillaud, J. L.; Marichal, C.; Soulard, M.; Patarin, J. Pure silica Chabazite molecular spring: A structural study on water intrusion-extrusion processes. J. Phys. Chem. B 2008, 112, 7257–7266.
Tzanis, L.; Trzpit, M.; Soulard, M.; Patarin, J. Energetic performances of channel and cage-type zeosils. J. Phys. Chem. C 2012, 116, 20389–20395.
Tzanis, L.; Trzpit, M.; Soulard, M.; Patarin, J. High pressure water intrusion investigation of pure silica 1D channel AFI, MTW and TON-type zeolites. Micropor. Mesopor. Mater. 2011, 146, 119–126.
Khay, I.; Tzanis, L.; Daou, T. J.; Nouali, H.; Ryzhikov, A.; Patarin, J. Energetic behavior of the pure silica ITQ-12 (ITW) zeolite under high pressure water intrusion. Phys. Chem. Chem. Phys. 2013, 15, 20320–20325.
Grosu, Y.; Li, M.; Peng, Y. L.; Luo, D.; Li, D.; Faik, A.; Nedelec, J. M.; Grolier, J. P. A highly stable nonhysteretic {Cu2(tebpz) MOF+water} molecular spring. ChemPhysChem 2016, 17, 3359–3364.
Park, K. S.; Ni, Z.; Côté, A. P.; Choi, J. Y.; Huang, R. D.; Uribe-Romo, F. J.; Chae, H. K.; O’Keeffe, M.; Yaghi, O. M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191.
Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O’Keeffe, M.; Yaghi, O. M. High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture. Science 2008, 319, 939–943.
Ortiz, G.; Nouali, H.; Marichal, C.; Chaplais, G.; Patarin, J. Energetic performances of the metal-organic framework ZIF-8 obtained using high pressure water intrusion-extrusion experiments. Phys. Chem. Chem. Phys. 2013, 15, 4888–4891.
Sun, Y. T.; Li, Y. B.; Tan, J. C. Framework flexibility of ZIF-8 under liquid intrusion: Discovering time-dependent mechanical response and structural relaxation. Phys. Chem. Chem. Phys. 2018, 20, 10108–10113.
Hu, Y.; Kazemian, H.; Rohani, S.; Huang, Y. N.; Song, Y. In situ high pressure study of ZIF-8 by FTIR spectroscopy. Chem. Commun. 2011, 47, 12694–12696.
Chen, S. L.; Li, X.; Dong, E. L.; Lv, H.; Yang, X. B.; Liu, R.; Liu, B. B. Intrinsic and extrinsic responses of ZIF-8 under high pressure: A combined Raman and X-ray diffraction investigation. J. Phys. Chem. C 2019, 123, 29693–29707.
Tortora, M.; Zajdel, P.; Lowe, A. R.; Chorążewski, M.; Leão, J. B.; Jensen, G. V.; Bleuel, M.; Giacomello, A.; Casciola, C. M.; Meloni, S. et al. Giant negative compressibility by liquid intrusion into superhydrophobic flexible nanoporous frameworks. Nano Lett. 2021, 21, 2848–2853.
Zajdel, P.; Madden, D. G.; Babu, R.; Tortora, M.; Mirani, D.; Tsyrin, N. N.; Bartolomé, L.; Amayuelas, E.; Fairen-Jimenez, D.; Lowe, A. R. et al. Turning molecular springs into Nano-shock absorbers: The effect of macroscopic morphology and crystal size on the dynamic hysteresis of water intrusion-extrusion into-from hydrophobic nanopores. ACS Appl. Mater. Interfaces 2022, 14, 26699–26713.
Sun, Y. T.; Rogge, S. M. J.; Lamaire, A.; Vandenbrande, S.; Wieme, J.; Siviour, C. R.; Van Speybroeck, V.; Tan, J. C. High-rate nanofluidic energy absorption in porous zeolitic frameworks. Nat. Mater. 2021, 20, 1015–1023.
Borjigin, T.; Sun, F. X.; Zhang, J. L.; Cai, K.; Ren, H.; Zhu, G. S. A microporous metal-organic framework with high stability for GC separation of alcohols from water. Chem. Commun. 2012, 48, 7613–7615.
Sun, Y. T.; Li, Y. B.; Tan, J. C. Liquid intrusion into zeolitic imidazolate framework-7 nanocrystals: Exposing the roles of phase transition and gate opening to enable energy absorption applications. ACS Appl. Mater. Interfaces 2018, 10, 41831–41838.
Chakraborty, S.; Kumar, H.; Dasgupta, C.; Maiti, P. K. Confined water: Structure, dynamics, and thermodynamics. Acc. Chem. Res. 2017, 50, 2139–2146.
Zhou, T. C.; Bai, P.; Siepmann, J. I.; Clark, A. E. Deconstructing the confinement effect upon the organization and dynamics of water in hydrophobic nanoporous materials: Lessons learned from zeolites. J. Phys. Chem. C 2017, 121, 22015–22024.
Paulo, G.; Gubbiotti, A.; Grosu, Y.; Meloni, S.; Giacomello, A. The impact of secondary channels on the wetting properties of interconnected hydrophobic nanopores. Commun. Phys. 2023, 6, 21.
Bushuev, Y. G.; Grosu, Y.; Chorążewski, M. A.; Meloni, S. Subnanometer topological tuning of the liquid intrusion/extrusion characteristics of hydrophobic micropores. Nano Lett. 2022, 22, 2164–2169.
Saliba, D.; Ammar, M.; Rammal, M.; Al-Ghoul, M.; Hmadeh, M. Crystal growth of ZIF-8, ZIF-67, and their mixed-metal derivatives. J. Am. Chem. Soc. 2018, 140, 1812–1823.
Cai, H. D.; Zhang, L. L.; Xu, J. S.; Huang, J. H.; Wei, X. L.; Wang, L.; Song, Z. Y.; Long, W. Cobalt-free La0.5Sr0.5Fe0.9Mo0.1O3−δ electrode for symmetrical SOFC running on H2 and CO fuels. Electrochim. Acta. 2019, 320, 134642.
Xu, X. L.; Zhang, X. M.; Xia, Z. X.; Sun, R. L.; Li, H. Q.; Wang, J. H.; Yu, S. S.; Wang, S. L.; Sun, G. Q. Solid phase microwave-assisted fabrication of Fe-doped ZIF-8 for single-atom Fe−N−C electrocatalysts on oxygen reduction. J. Energy Chem. 2021, 54, 579–586.
Benzaqui, M.; Semino, R.; Menguy, N.; Carn, F.; Kundu, T.; Guigner, J. M.; McKeown, N. B.; Msayib, K. J.; Carta, M.; Malpass-Evans, R. et al. Toward an understanding of the microstructure and interfacial properties of PIMs/ZIF-8 mixed matrix membranes. ACS Appl. Mater. Interfaces 2016, 8, 27311–27321.
Gao, M. Z.; Wang, J.; Rong, Z. H.; Shi, Q.; Dong, J. X. A combined experimental-computational investigation on water adsorption in various ZIFs with the SOD and RHO topologies. RSC Adv. 2018, 8, 39627–39634.
Skarmoutsos, I.; Eddaoudi, M.; Maurin, G. Highly efficient rare-earth-based metal-organic frameworks for water adsorption: A molecular modeling approach. J. Phys. Chem. C 2019, 123, 26989–26999.
Zhang, H.; Singer, S. J. Analysis of the subcritical carbon dioxide-water interface. J. Phys. Chem. A 2011, 115, 6285–6296.
Tang, Y. B.; Xie, S. J. Structure and dynamics of a water/methanol mixture confined in zeolitic imidazolate framework ZIF-8 from atomistic simulations. Phys. Chem. Chem. Phys. 2022, 24, 5220–5232.
Li, Z.; Zeng, H. C. Surface and bulk integrations of single-layered Au or Ag nanoparticles onto designated crystal planes {110} or {100} of ZIF-8. Chem. Mater. 2013, 25, 1761–1768.
Wang, M.; Liu, J. X.; Guo, C. M.; Gao, X. S.; Gong, C. H.; Wang, Y.; Liu, B.; Li, X. X.; Gurzadyan, G. G.; Sun, L. C. Metal-organic frameworks (ZIF-67) as efficient cocatalysts for photocatalytic reduction of CO2: The role of the morphology effect. J. Mater. Chem. A 2018, 6, 4768–4775.
Cravillon, J.; Nayuk, R.; Springer, S.; Feldhoff, A.; Huber, K.; Wiebcke, M. Controlling zeolitic imidazolate framework nano- and microcrystal formation: Insight into crystal growth by time-resolved in situ static light scattering. Chem. Mater. 2011, 23, 2130–2141.
Shen, K.; Zhang, L.; Chen, X. D.; Liu, L. M.; Zhang, D. L.; Han, Y.; Chen, J. Y.; Long, J. L.; Luque, R.; Li, Y. W. et al. Ordered macromicroporous metal-organic framework single crystals. Science 2018, 359, 206–210.
James, J. B.; Lin, Y. S. Kinetics of ZIF-8 Thermal decomposition in inert, oxidizing, and reducing environments. J. Phys. Chem. C 2016, 120, 14015–14026.
Zhu, R. M.; Ding, J. W.; Yang, J. P.; Pang, H.; Xu, Q.; Zhang, D. L.; Braunstein, P. Quasi-ZIF-67 for boosted oxygen evolution reaction catalytic activity via a low temperature calcination. ACS Appl. Mater. Interfaces 2020, 12, 25037–25041.
Zhao, Y. L.; Wei, Y. Y.; Lyu, L.; Hou, Q. Q.; Caro, J.; Wang, H. H. Flexible polypropylene-supported ZIF-8 membranes for highly efficient propene/propane separation. J. Am. Chem. Soc. 2020, 142, 20915–20919.
Mor, J.; Sharma, S. K.; Utpalla, P.; Bahadur, J.; Prakash, J.; Kumar, A.; Pujari, P. K. Pore architecture evolution and OER catalytic activity of hollow Co/Zn zeolitic imidazolate frameworks. Micropor. Mesopor. Mater. 2022, 335, 111814.
Ordoñez, M. J. C.; Balkus, K. J.; Ferraris, J. P.; Musselman, I. H. Molecular sieving realized with ZIF-8/matrimid® mixed-matrix membranes. J. Membr. Sci. 2010, 361, 28–37.
Tanaka, S.; Tanaka, Y. A simple step toward enhancing hydrothermal stability of ZIF-8. ACS Omega 2019, 4, 19905–19912.
Wu, C. H.; Xie, D. G.; Mei, Y. J.; Xiu, Z. F.; Poduska, K. M.; Li, D. C.; Xu, B.; Sun, D. F. Unveiling the thermolysis natures of ZIF-8 and ZIF-67 by employing in situ structural characterization studies. Phys. Chem. Chem. Phys. 2019, 21, 17571–17577.
Willems, T. F.; Rycroft, C. H.; Kazi, M.; Meza, J. C.; Haranczyk, M. Algorithms and tools for high-throughput geometry-based analysis of crystalline porous materials. Micropor. Mesopor. Mater. 2012, 149, 134–141.
Calero, S.; Gómez-Álvarez, P. Underlying adsorption mechanisms of water in hydrophobic and hydrophilic zeolite imidazolate frameworks: ZIF-71 and ZIF-90. J. Phys. Chem. C 2015, 119, 23774–23780.
Jayachandrababu, K. C.; Sholl, D. S.; Nair, S. Structural and mechanistic differences in mixed-linker zeolitic imidazolate framework synthesis by solvent assisted linker exchange and de novo routes. J. Am. Chem. Soc. 2017, 139, 5906–5915.
Krishna, R.; Van Baten, J. M. Water/alcohol mixture adsorption in hydrophobic materials: Enhanced water ingress caused by hydrogen bonding. ACS Omega 2020, 5, 28393–28402.
Mortada, B.; Chaplais, G.; Nouali, H.; Marichal, C.; Patarin, J. Phase transformations of metal-organic frameworks MAF-6 and ZIF-71 during intrusion-extrusion experiments. J. Phys. Chem. C 2019, 123, 4319–4328.
Gao, Y.; Li, M. Z.; Zhang, Y.; Lu, W. Y.; Xu, B. X. Spontaneous outflow efficiency of confined liquid in hydrophobic nanopores. Proc. Natl. Acad. Sci. USA 2020, 117, 25246–25253.
Mortada, B.; Chaplais, G.; Veremeienko, V.; Nouali, H.; Marichal, C.; Patarin, J. Energetic performances of ZIF-8 derivatives: Impact of the substitution (Me, Cl, or Br) on imidazolate linker. J. Phys. Chem. C 2018, 122, 3846–3855.
Desbiens, N.; Demachy, I.; Fuchs, A. H.; Kirsch-Rodeschini, H.; Soulard, M.; Patarin, J. Water condensation in hydrophobic nanopores. Angew. Chem., Int. Ed. 2005, 44, 5310–5313.
Ortiz, G.; Nouali, H.; Marichal, C.; Chaplais, G.; Patarin, J. Energetic performances of “ZIF-71-aqueous solution” systems: A perfect shock-absorber with water. J. Phys. Chem. C 2014, 118, 21316–21322.
Ortiz, G.; Nouali, H.; Marichal, C.; Chaplais, G.; Patarin, J. Versatile energetic behavior of ZIF-8 upon high pressure intrusion-extrusion of aqueous electrolyte solutions. J. Phys. Chem. C 2014, 118, 7321–7328.
Sharma, M.; Wu, Y. D.; Car, R. Ab initio molecular dynamics with maximally localized wannier functions. Int. J. Quantum Chem. 2003, 95, 821–829.
Soper, A. K.; Ricci, M. A. Structures of high-density and low-density water. Phys. Rev. Lett. 2000, 84, 2881–2884.
Zhang, L.; Zheng, B.; Gao, Y.; Wang, L. L.; Wang, J. L.; Duan, X. B. Confined water vapor in ZIF-8 nanopores. ACS Omega 2022, 7, 64–69.
Tang, F. J.; Li, Z. L.; Zhang, C. Y.; Louie, S. G.; Car, R.; Qiu, D. Y.; Wu, X. F. Many-body effects in the X-ray absorption spectra of liquid water. Proc. Natl. Acad. Sci. USA 2022, 119, e2201258119.
Calero, S.; Gómez-Álvarez, P. Effect of the confinement and presence of cations on hydrogen bonding of water in LTA-type zeolite. J. Phys. Chem. C 2014, 118, 9056–9065.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 22178109 and 21878097) and the Natural Science Foundation of Shanghai (No. 21ZR1417700). D. W. acknowledges the institutional funds from the Gene and Linda Voiland School of Chemical Engineering and Bioengineering, and the Alexandra Navrotsky Institute for Experimental Thermodynamics at Washington State University.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
12274_2023_5967_MOESM1_ESM.pdf
Elucidating and manipulating pressure-induced water intrusion–extrusion in tunable hydrophobic Co/Zn bimetallic ZIFs: Roles of pore size and hydrogen bond
Rights and permissions
About this article
Cite this article
Fang, D., Liu, C., Chen, Y. et al. Elucidating and manipulating pressure-induced water intrusion–extrusion in tunable hydrophobic Co/Zn bimetallic ZIFs: Roles of pore size and hydrogen bond. Nano Res. 17, 344–353 (2024). https://doi.org/10.1007/s12274-023-5967-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-023-5967-5