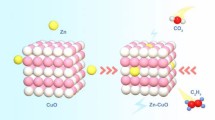
Abstract
Electrochemical NO-to-NH3 under ambient conditions could be a viable alternative having advantages in terms of energy consumption and exhaust gas recycling of NO, replacing a traditional ammonia synthesis method of the Haber–Bosch process. In synthesizing boron (B-) and nitrogen (N-) co-doped carbon nanotube (CNT) based gold (Au) catalysts, B-dopants elevate the conductivity of carbon nanotube by sp2 hybridization on graphene and implant B–N domains within the graphene layer, and result in facilitating the embedding amount of Au accompanied by high dispersibility with low particle size. Theoretical density functional theory (DFT) calculations elucidate that the electron cloud transmitted from B-dopant to the active site of Au induces the Lewis acidic site, and the O-distal pathway occurs following a spontaneous reaction. Increment of the electron-deficient B-doping area accompanied by N-defects and B–O edges retains the major valence state of Au as Auδ+, and suppresses hydrogen evolution reaction (HER) by repulsing the hindrance of H⋆. This record exhibits the highest faradaic efficiency (FE) of 94.7%, and NH3 yield rate of 1877.4 µg·h−1·mgcat−1, which is the optimal yield over energy consumption in the field of the ambient reduction of aqueous NO.

Similar content being viewed by others
References
Ye, B.; Jeong, B.; Lee, M. J.; Kim, T. H.; Park, S. S.; Jung, J.; Lee, S.; Kim, H. D. Recent trends in vanadium-based SCR catalysts for NOx reduction in industrial applications: Stationary sources. Nano Converg. 2022, 9, 51.
Lee, T.; Bai, H. Low temperature selective catalytic reduction of NOX with NH3 over Mn-based catalyst: A review. AIMS Environmen. Sci. 2016, 3, 261–289.
Liang, J.; Chen, H. Y.; Mou, T.; Zhang, L. C.; Lin, Y. T.; Yue, L. C.; Luo, Y. S.; Liu, Q.; Li, N.; Alshehri, A. A. et al. Coupling denitrification and ammonia synthesis via selective electrochemical reduction of nitric oxide over Fe2O3 nanorods. J. Mater. Chem. A 2022, 10, 6454–6462.
Alves, L.; Holz, L. I. V.; Fernandes, C.; Ribeirinha, P.; Mendes, D.; Fagg, D. P.; Mendes, A. A comprehensive review of NOx and N2O mitigation from industrial streams. Renew. Sust. Energy Rev. 2022, 155, 111916.
Koebel, M.; Madia, G.; Elsener, M. Selective catalytic reduction of NO and NO2 at low temperatures. Catal. Today 2002, 73, 239–247.
Sharif, H. M. A.; Mahmood, N.; Wang, S. Y.; Hussain, I.; Hou, Y. N.; Yang, L. H.; Zhao, X.; Yang, B. Recent advances in hybrid wet scrubbing techniques for NOx and SO2 removal: State of the art and future research. Chemosphere 2021, 273, 129695.
Zhang, Y. Y.; Cao, G. J.; Yang, X. Advances in De-NOx methods and catalysts for direct catalytic decomposition of NO: A review. Energy Fuels 2021, 35, 6443–6464.
Chen, H. H.; Zhang, C. Q.; Sheng, L.; Wang, M. M.; Fu, W.; Gao, S.; Zhang, Z. R.; Chen, S. Q.; Si, R.; Wang, L. Z. et al. Copper single-atom catalyst as a high-performance electrocatalyst for nitrate-ammonium conversion. J. Hazard. Mater. 2022, 434, 128892.
Tang, C.; Qiao, S. Z. How to explore ambient electrocatalytic nitrogen reduction reliably and insightfully. Chem. Soc. Rev. 2019, 48, 3166–3180.
Chen, J. G.; Crooks, R. M.; Seefeldt, L. C.; Bren, K. L.; Bullock, R. M.; Darensbourg, M. Y.; Holland, P. L.; Hoffman, B.; Janik, M. J.; Jones, A. K. et al. Beyond fossil fuel-driven nitrogen transformations. Science 2018, 360, eaar6611.
Galloway, J. N.; Townsend, A. R.; Erisman, J. W.; Bekunda, M.; Cai, Z. C.; Freney, J. R.; Martinelli, L. A.; Seitzinger, S. P.; Sutton, M. A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892.
Lv, C. D.; Liu, J. W.; Lee, C.; Zhu, Q.; Xu, J. W.; Pan, H. G.; Xue, C.; Yan, Q. Y. Emerging p-block-element-based electrocatalysts for sustainable nitrogen conversion. ACS Nano 2022, 16, 15512–15527.
van der Ham, C. J. M.; Koper, M. T. M.; Hetterscheid, D. G. H. Challenges in reduction of dinitrogen by proton and electron transfer. Chem. Soc. Rev. 2014, 43, 5183–5191.
Wu, T. T.; Fan, W. J.; Zhang, Y.; Zhang, F. X. Electrochemical synthesis of ammonia: Progress and challenges. Mater. Today Phys. 2021, 16, 100310.
Qing, G.; Ghazfar, R.; Jackowski, S. T.; Habibzadeh, F.; Ashtiani, M. M.; Chen, C. P.; Smith III, M. R.; Hamann, T. W. Recent advances and challenges of electrocatalytic N2 reduction to ammonia. Chem. Rev. 2020, 120, 5437–5516.
Chen, H. J.; Liang, J.; Li, L.; Zheng, B. Z.; Feng, Z. S.; Xu, Z. Q.; Luo, Y. L.; Liu, Q.; Shi, X. F.; Liu, Y. et al. Ti2O3 nanoparticles with Ti3+ sites toward efficient NH3 electrosynthesis under ambient conditions. ACS Appl. Mater. Interfaces 2021, 13, 41715–41722.
Li, S. X.; Wu, Y. M.; Liu, Q.; Li, B. H.; Li, T. S.; Zhao, H. T.; Alshehri, A. A.; Alzahrani, K. A.; Luo, Y. L.; Li, L. et al. CuS concave polyhedral superstructures enabled efficient N2 electroreduction to NH3 at ambient conditions. Inorg. Chem. Front. 2021, 8, 3105–3110.
Liu, S. S.; Qian, T.; Wang, M. F.; Ji, H. Q.; Shen, X. W.; Wang, C.; Yan, C. L. Proton-filtering covalent organic frameworks with superior nitrogen penetration flux promote ambient ammonia synthesis. Nat. Catal. 2021, 4, 322–331.
Yang, B.; Ding, W. L.; Zhang, H. H.; Zhang, S. J. Recent progress in electrochemical synthesis of ammonia from nitrogen: Strategies to improve the catalytic activity and selectivity. Energy Environ. Sci. 2021, 14, 672–687.
Guo, C. X.; Ran, J. R.; Vasileff, A.; Qiao, S. Z. Rational design of electrocatalysts and photo (electro) catalysts for nitrogen reduction to ammonia (NH3) under ambient conditions. Energy Environ. Sci. 2018, 11, 45–56.
Peng, X. Y.; Mi, Y. Y.; Bao, H. H.; Liu, Y. F.; Qi, D. F.; Qiu, Y.; Zhuo, L. C.; Zhao, S. Z.; Sun, J. Q.; Tang, X. L. et al. Ambient electrosynthesis of ammonia with efficient denitration. Nano Energy 2020, 78, 105321.
Yang, J.; Qi, H. F.; Li, A. Q.; Liu, X. Y.; Yang, X. F.; Zhang, S. X.; Zhao, Q.; Jiang, Q. K.; Su, Y.; Zhang, L. L. et al. Potential-driven restructuring of Cu single atoms to nanoparticles for boosting the electrochemical reduction of nitrate to ammonia. J. Am. Chem. Soc. 2022, 144, 12062–12071.
Cheon, S.; Kim, W. J.; Kim, D. Y.; Kwon, Y.; Han, J. I. Electro-synthesis of ammonia from dilute nitric oxide on a gas diffusion electrode. ACS Energy Lett. 2022, 7, 958–965.
Long, J.; Chen, S. M.; Zhang, Y. L.; Guo, C. X.; Fu, X. Y.; Deng, D. H.; Xiao, J. P. Direct electrochemical ammonia synthesis from nitric oxide. Angew. Chem., Int. Ed. 2020, 59, 9711–9718.
Ko, B. H.; Hasa, B.; Shin, H.; Zhao, Y. R.; Jiao, F. Electrochemical reduction of gaseous nitrogen oxides on transition metals at ambient conditions. J. Am. Chem. Soc. 2022, 144, 1258–1266.
Rhimi, B.; Padervand, M.; Jouini, H.; Ghasemi, S.; Bahnemann, D. W.; Wang, C. Recent progress in NOx photocatalytic removal: Surface/interface engineering and mechanistic understanding. J. Environ. Chem. Eng. 2022, 1, 108566.
Herzing, A. A.; Kiely, C. J.; Carley, A. F.; Landon, P.; Hutchings, G. J. Identification of active gold nanoclusters on iron oxide supports for CO oxidation. Science 2008, 321, 1331–1335.
Rosca, V.; Beltramo, G. L.; Koper, M. T. Reduction of NO adlayers on Pt (110) and Pt (111) in acidic media: Evidence for adsorption site-specific reduction. Langmuir 2005, 21, 1448–1456.
Rosca, V.; Koper, M. T. M. Mechanism of electrocatalytic reduction of nitric oxide on Pt (100). J. Phys. Chem. B 2005, 109, 16750–16759.
Liu, H.; Xiang, K. S.; Yang, B. T.; Xie, X. F.; Wang, D. L.; Zhang, C.; Liu, Z. L.; Yang, S.; Liu, C.; Zou, J. P. et al. The electrochemical selective reduction of NO using CoSe2@CNTs hybrid. Environ. Sci. Pollut. Res. 2017, 24, 14249–14258.
Kim, D. H.; Ringe, S.; Kim, H.; Kim, S.; Kim, B.; Bae, G.; Oh, H. S.; Jaouen, F.; Kim, W.; Kim, H. et al. Selective electrochemical reduction of nitric oxide to hydroxylamine by atomically dispersed iron catalyst. Nat. Commun. 2021, 12, 1856.
Li, J. C.; Li, M.; An, N.; Zhang, S.; Song, Q. N.; Yang, Y. L.; Li, J.; Liu, X. Boosted ammonium production by single cobalt atom catalysts with high Faradic efficiencies. Proc. Natl. Acad. Sci. USA 2022, 119, e2123450119.
Wu, Q.; Wang, H.; Shen, S. Y.; Huang, B. B.; Dai, Y.; Ma, Y. D. Efficient nitric oxide reduction to ammonia on a metal-free electrocatalyst. J. Mater. Chem. A 2021, 9, 5434–5441.
Sa, Y. J.; Park, C.; Jeong, H. Y.; Park, S. H.; Lee, Z.; Kim, K. T.; Park, G. G.; Joo, S. H. Carbon nanotubes/heteroatom-doped carbon core-sheath nanostructures as highly active, metal-free oxygen reduction electrocatalysts for alkaline fuel cells. Angew. Chem. 2014, 126, 4186–4190.
Choe, J.; Sun, W. T.; Ombrello, T.; Carter, C. Plasma assisted ammonia combustion: Simultaneous NOx reduction and flame enhancement. Combust. Flame 2021, 228, 430–432.
Ren, Z. B.; Zhang, H. N.; Wang, S. H.; Huang, B. B.; Dai, Y.; Wei, W. Nitric oxide reduction reaction for efficient ammonia synthesis on topological nodal-line semimetal Cu2Si monolayer. J. Mater. Chem. A 2022, 10, 8568–8577.
Chen, R.; Fang, X.; Li, Z.; Liu, Z. Selective catalytic reduction of NO, with NH3 over a novel MOF-derived MnOx catalyst. Appl. Catal. A: Gen. 2022, 643, 118754.
Tabassum, H.; Guo, W. H.; Meng, W.; Mahmood, A.; Zhao, R.; Wang, Q. F.; Zou, R. Q. Metal-organic frameworks derived cobalt phosphide architecture encapsulated into B/N Co-doped graphene nanotubes for all pH value electrochemical hydrogen evolution. Adv. Energy Mater. 2017, 7, 1601671.
Cui, C. X.; Gao, Y.; Li, J.; Yang, C.; Liu, M.; Jin, H. L.; Xia, Z. H.; Dai, L. M.; Lei, Y.; Wang, J. C. et al. Origins of boosted charge storage on heteroatom-doped carbons. Angew. Chem. 2020, 132, 8002–8007.
Yan, D. F.; Dou, S.; Tao, L.; Liu, Z. J.; Liu, Z. G.; Huo, J.; Wang, S. Y. Electropolymerized supermolecule derived N, P co-doped carbon nanofiber networks as a highly efficient metal-free electrocatalyst for the hydrogen evolution reaction. J. Mater. Chem. A 2016, 4, 13726–13730.
Tabassum, H.; Zou, R. Q.; Mahmood, A.; Liang, Z. B.; Guo, S. J. A catalyst-free synthesis of B, N co-doped graphene nanostructures with tunable dimensions as highly efficient metal free dual electrocatalysts. J. Mater. Chem. A 2016, 4, 16469–16475.
Chen, Z. P.; Mitchell, S.; Vorobyeva, E.; Leary, R. K.; Hauert, R.; Furnival, T.; Ramasse, Q. M.; Thomas, J. M.; Midgley, P. A.; Dontsova, D. et al. Stabilization of single metal atoms on graphitic carbon nitride. Adv. Funct. Mater. 2017, 27, 1605785.
Zhang, X. F.; Yan, P. Q.; Xu, J. K.; Li, F.; Herold, F.; Etzold, B. J. M.; Wang, P.; Su, D. S.; Lin, S.; Qi, W. et al. Methanol conversion on borocarbonitride catalysts: Identification and quantification of active sites. Sci. Adv. 2020, 6, eaba5778.
Wang, S. Y.; Iyyamperumal, E.; Roy, A.; Xue, Y. H.; Yu, D. S.; Dai, L. M. Vertically aligned BCN nanotubes as efficient metalfree electrocatalysts for the oxygen reduction reaction: A synergetic effect by co-doping with boron and nitrogen. Angew. Chem., Int. Ed. 2011, 50, 11756–11760.
Wang, W. L.; Bai, X. D.; Liu, K. H.; Xu, Z.; Golberg, D.; Bando, Y.; Wang, E. G. Direct synthesis of B-C-N single-walled nanotubes by bias-assisted hot filament chemical vapor deposition. J. Am. Chem. Soc. 2006, 128, 6530–6531.
Zheng, Y.; Jiao, Y.; Ge, L.; Jaroniec, M.; Qiao, S. Z. Two-step boron and nitrogen doping in graphene for enhanced synergistic catalysis. Angew. Chem. 2013, 125, 3192–3198.
Shi, L.; Yin, Y.; Wang, S. B.; Sun, H. Q. Rational catalyst design for N2 reduction under ambient conditions: Strategies toward enhanced conversion efficiency. ACS Catal. 2020, 10, 6870–6899.
Tabassum, H.; Qu, C.; Cai, K. T.; Aftab, W.; Liang, Z. B.; Qiu, T. J.; Mahmood, A.; Meng, W.; Zou, R. Q. Large-scale fabrication of BCN nanotube architecture entangled on a three-dimensional carbon skeleton for energy storage. J. Mater. Chem. A 2018, 6, 21225–21230.
Choi, C. H.; Park, S. H.; Woo, S. I. Binary and ternary doping of nitrogen, boron, and phosphorus into carbon for enhancing electrochemical oxygen reduction activity. ACS Nano 2012, 6, 7084–7091.
Guo, F. S.; Yang, P. J.; Pan, Z. M.; Cao, X. N.; Xie, Z. L.; Wang, X. C. Carbon-doped BN nanosheets for the oxidative dehydrogenation of ethylbenzene. Angew. Chem. 2017, 129, 8343–8347.
Légaré, M. A.; Bélanger-Chabot, G.; Dewhurst, R. D.; Welz, E.; Krummenacher, I.; Engels, B.; Braunschweig, H. Nitrogen fixation and reduction at boron. Science 2018, 359, 896–900.
Yu, X. M.; Han, P.; Wei, Z. X.; Huang, L. S.; Gu, Z. X.; Peng, S. J.; Ma, J. M.; Zheng, G. F. Boron-doped graphene for electrocatalytic N2 reduction. Joule 2018, 2, 1610–1622.
Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S. Z. Origin of the electrocatalytic oxygen reduction activity of graphene-based catalysts: A roadmap to achieve the best performance. J. Am. Chem. Soc. 2014, 136, 4394–4403.
Panchakarla, L. S.; Subrahmanyam, K. S.; Saha, S. K.; Govindaraj, A.; Krishnamurthy, H. R.; Waghmare, U. V.; Rao, C. N. R. Synthesis, structure, and properties of boron-and nitrogen-doped graphene. Adv. Mater. 2009, 21, 4726–4730.
Lu, Z. S.; Lv, P.; Yang, Z. X.; Li, S.; Ma, D. W.; Wu, R. Q. A promising single atom catalyst for CO oxidation: Ag on boron vacancies of h-BN sheets. Phys. Chem. Chem. Phys. 2017, 19, 16795–16805.
Liu, Z. M.; Gao, D. Z.; Hu, L. N.; Liu, F.; Liu, H.; Li, Y.; Zhang, J.; Xue, Y. M.; Tang, C. C. Metal-free boron-rich borocarbonitride catalysts for high-efficient oxygen reduction to produce hydrogen peroxide. ChemistrySelect 2022, 7, e202104203.
Feng, Y.; Yao, J. F. Design of melamine sponge-based three-dimensional porous materials toward applications. Ind. Eng. Chem. Res. 2018, 57, 7322–7330.
Chen, X.; Liu, Y.; Ke, X. X.; Weerasooriya, R.; Li, H.; Wang, L. C.; Wu, Y. C. A green method to synthesize AuNPs/mpg-C3N4 nanocomposites for constructing anti-interference electrochemical sensing interface toward methylmercury. J. Alloys Compd. 2021, 853, 157365.
B. V.; Usachov, D. Y.; Fedorov, A. V.; Marangoni, T.; Haberer, D.; Tresca, C.; Profeta, G.; Caciuc, V.; Tsukamoto, S.; Atodiresei, N. et al. Boron-doped graphene nanoribbons: Electronic structure and Raman fingerprint. ACS Nano 2018, 12, 7571–7582.
Shi, M. M.; Bao, D.; Wulan, B. R.; Li, Y. H.; Zhang, Y. F.; Yan, J. M.; Jiang, Q. Au sub-nanoclusters on TiO2 toward highly efficient and selective electrocatalyst for N2 conversion to NH3 at ambient conditions. Adv. Mater. 2017, 29, 1606550.
He, H. M.; Zhu, Q. Q.; Yan, Y.; Zhang, H. W.; Han, Z. Y.; Sun, H. M.; Chen, J.; Li, C. P.; Zhang, Z. H.; Du, M. Metal-organic framework supported Au nanoparticles with organosilicone coating for high-efficiency electrocatalytic N2 reduction to NH3. Appl. Catal. B:Environ. 2022, 302, 120840.
Zhao, X.; Yang, Z. Q.; Kuklin, A. V.; Baryshnikov, G. V.; Ågren, H.; Zhou, X. H.; Zhang, H. B. Efficient ambient electrocatalytic ammonia synthesis by nanogold triggered via boron clusters combined with carbon nanotubes. ACS Appl. Mater. Interfaces 2020, 12, 42821–42831.
Li, W. Y.; Zhang, C.; Han, M. M.; Ye, Y. X.; Zhang, S. B.; Liu, Y. Y.; Wang, G. Z.; Liang, C. H.; Zhang, H. M. Ambient electrosynthesis of ammonia using core-shell structured Au@C catalyst fabricated by one-step laser ablation technique. ACS Appl. Mater. Interfaces 2019, 11, 44186–44195.
Deshpande, S.; Greeley, J. First-principles analysis of coverage, ensemble, and solvation effects on selectivity trends in NO electroreduction on Pt3Sn alloys. ACS Catal. 2020, 10, 9320–9327.
Niu, H.; Zhang, Z. F.; Wang, X. T.; Wan, X. H.; Kuai, C. G.; Guo, Y. Z. A feasible strategy for identifying single-atom catalysts toward electrochemical NO-to-NH3 conversion. Small 2021, 17, 2102396.
Xiong, Y. H.; Li, Y. T.; Wan, S. P.; Yu, Y.; Zhang, S. L.; Zhong, Q. Ferrous-based electrolyte for simultaneous NO absorption and electroreduction to NH3 using Au/rGO electrode. J. Hazard. Mater. 2022, 430, 128451.
Liang, J.; Liu, Q.; Alshehri, A. A.; Sun, X. P. Recent advances in nanostructured heterogeneous catalysts for N-cycle electrocatalysis. Nano Res. Energy 2022, 1, e9120010.
Choi, J.; Du, H. L.; Nguyen, C. K.; Suryanto, B. H. R.; Simonov, A. N.; MacFarlane, D. R. Electroreduction of nitrates, nitrites, and gaseous nitrogen oxides: A potential source of ammonia in dinitrogen reduction studies. ACS Energy Lett. 2020, 5, 2095–2097.
de Vooys, A. C. A.; Koper, M. T. M.; van Santen, R. A.; van Veen, J. A. R. Mechanistic study on the electrocatalytic reduction of nitric oxide on transition-metal electrodes. J. Catal. 2001, 202, 387–394.
Qin, Q.; Heil, T.; Antonietti, M.; Oschatz, M. Single-site gold catalysts on hierarchical N-doped porous noble carbon for enhanced electrochemical reduction of nitrogen. Small Methods 2018, 2, 1800202.
Xue, Z. H.; Zhang, S. N.; Lin, Y. X.; Su, H.; Zhai, G. Y.; Han, J. T.; Yu, Q. Y.; Li, X. H.; Antonietti, M.; Chen, J. S. Electrochemical reduction of N2 into NH3 by donor-acceptor couples of Ni and Au nanoparticles with a 67.8% Faradaic efficiency. J. Am. Chem. Soc. 2019, 141, 14976–14980.
Hu, Q.; Gao, K. R.; Wang, X. D.; Zheng, H. J.; Cao, J. Y.; Mi, L. R.; Huo, Q. H.; Yang, H. P.; Liu, J. H.; He, C. X. Subnanometric Ru clusters with upshifted D band center improve performance for alkaline hydrogen evolution reaction. Nat. Commun. 2022, 13, 3958.
Liang, X.; Fu, N. H.; Yao, S. C.; Li, Z.; Li, Y. D. The progress and outlook of metal single-atom-site catalysis. J. Am. Chem. Soc. 2022, 144, 18155–18174.
Liu, S. F.; Xu, W.; Niu, Y. M.; Zhang, B. S.; Zheng, L. R.; Liu, W.; Li, L.; Wang, J. H. Ultrastable Au nanoparticles on Titania through an encapsulation strategy under oxidative atmosphere. Nat. Commun. 2019, 10, 5790.
Jin, H. Y.; Wang, J.; Su, D. F.; Wei, Z. Z.; Pang, Z. F.; Wang, Y. In situ cobalt-cobalt oxide/N-doped carbon hybrids as superior bifunctional electrocatalysts for hydrogen and oxygen evolution. J. Am. Chem. Soc. 2015, 137, 2688–2694.
Zhang, H.; Zhou, Y.; Li, C. G.; Chen, S. L.; Liu, L.; Liu, S. W.; Yao, H. M.; Hou, H. Q. Porous nitrogen doped carbon foam with excellent resilience for self-supported oxygen reduction catalyst. Carbon 2015, 95, 388–395.
Chen, J. Z.; Xu, J. L.; Zhou, S.; Zhao, N.; Wong, C. P. Nitrogen-doped hierarchically porous carbon foam: A free-standing electrode and mechanical support for high-performance supercapacitors. Nano Energy 2016, 25, 193–202.
Chen, L.; Du, R.; Zhu, J. H.; Mao, Y. Y.; Xue, C.; Zhang, N.; Hou, Y. L.; Zhang, J.; Yi, T. Three-dimensional nitrogen-doped graphene nanoribbons aerogel as a highly efficient catalyst for the oxygen reduction reaction. Small 2015, 11, 1423–1429.
Pang, Y. Y.; Wang, K.; Xie, H.; Sun, Y.; Titirici, M. M.; Chai, G. L. Mesoporous carbon hollow spheres as efficient electrocatalysts for oxygen reduction to hydrogen peroxide in neutral electrolytes. ACS Catal. 2020, 10, 7434–7442.
Chen, S. C.; Chen, Z. H.; Siahrostami, S.; Kim, T. R.; Nordlund, D.; Sokaras, D.; Nowak, S.; To, J. W.; Higgins, D.; Sinclair, R. et al. Defective carbon-based materials for the electrochemical synthesis of hydrogen peroxide. ACS Sustain. Chem. Eng. 2018, 6, 311–317.
Yin, H. B.; Chen, Z.; Xiong, S. C.; Chen, J. J.; Wang, C. Z.; Wang, R.; Kuwahara, Y.; Luo, J. S.; Yamashita, H.; Peng, Y. et al. Alloying effect-induced electron polarization drives nitrate electroreduction to ammonia. Chem Catal. 2021, 1, 1088–1103.
Zhang, L. C.; Zhou, Q.; Liang, J.; Yue, L. C.; Li, T. S.; Luo, Y. S.; Liu, Q.; Li, N.; Tang, B.; Gong, F. et al. Enhancing electrocatalytic NO reduction to NH3 by the CoS nanosheet with sulfur vacancies. Inorg. Chem. 2022, 61, 8096–8102.
Frear, D. S.; Burrell, R. C. Spectrophotometric method for determining hydroxylamine reductase activity in higher plants. Anal. Chem. 1955, 27, 1664–1665.
Ye, S. H.; Luo, F. Y.; Xu, T. T.; Zhang, P. Y.; Shi, H. D.; Qin, S. Q.; Wu, J. P.; He, C. X.; Ouyang, X. P.; Zhang, Q. L. et al. Boosting the alkaline hydrogen evolution of Ru nanoclusters anchored on B/N-doped graphene by accelerating water dissociation. Nano Energy 2020, 68, 104301.
Mannan, A.; Hirano, Y.; Quitain, A. T.; Koinuma, M.; Kida, T. Graphene oxide to B, N co-doped graphene through tris-dimethylaminoborane complex by hydrothermal implantation. Am. J. Mater. Sci. 2019, 9, 22–28.
Fedoseeva, Y. V.; Lobiak, E. V.; Shlyakhova, E. V.; Kovalenko, K. A.; Kuznetsova, V. R.; Vorfolomeeva, A. A.; Grebenkina, M. A.; Nishchakova, A. D.; Makarova, A. A.; Bulusheva, L. G. et al. Hydrothermal activation of porous nitrogen-doped carbon materials for electrochemical capacitors and sodium-ion batteries. Nanomaterials 2020, 10, 2163.
Domnich, V.; Reynaud, S.; Haber, R. A.; Chhowalla, M. Boron carbide: Structure, properties, and stability under stress. J. Am Ceram Soc. 2011, 94, 3605–3628.
Chakrabarty, K.; Chen, W. C.; Baker, P. A.; Vijayan, V. M.; Chen, C. C.; Catledge, S. A. Superhard boron-rich boron carbide with controlled degree of crystallinity. Materials 2020, 13, 3622.
Werheit, H.; Au, T.; Schmechel, R.; Shalamberidze, S. O.; Kalandadze, G. I.; Eristavi, A. M. IR-active phonons and structure elements of isotope-enriched boron carbide. J. Solid State Chem. 2000, 154, 79–86.
He, Y. H.; Guo, H.; Hwang, S.; Yang, X. X.; He, Z. Z.; Braaten, J.; Karakalos, S.; Shan, W. T.; Wang, M. Y.; Zhou, H. et al. Single cobalt sites dispersed in hierarchically porous nanofiber networks for durable and high-power PGM-free cathodes in fuel cells. Adv. Mater. 2020, 32, 2003577.
Lin, W. W.; Chen, H.; Lin, G. B.; Yao, S. Y.; Zhang, Z. H.; Qi, J. Z.; Jing, M. Z.; Song, W. Y.; Li, J.; Liu, X. et al. Creating frustrated lewis pairs in defective boron carbon nitride for electrocatalytic nitrogen reduction to ammonia. Angew. Chem., Int. Ed. 2022, 61, e202207807.
Zhang, J. S.; Zhang, M. W.; Sun, R. Q.; Wang, X. C. A facile band alignment of polymeric carbon nitride semiconductors to construct isotype heterojunctions. Angew. Chem. 2012, 124, 10292–10296.
Yang, Y. T.; Wang, Y.; Wang, X.; Chen, S. Q.; Duan, L. M.; Zhang, W.; Li, W. F.; Liu, J. H. Tailoring electron-riched boron sites in BCN for nitrogen fixation via alternate mechanism. Adv. Mater. Interfaces 2022, 9, 2101842.
Huang, C. J.; Chen, C.; Zhang, M. W.; Lin, L. H.; Ye, X. X.; Lin, S.; Antonietti, M.; Wang, X. C. Carbon-doped BN nanosheets for metal-free photoredox catalysis. Nat. Commun. 2015, 6, 7698.
Tabbal, M.; Christidis, T.; Isber, S.; Mérel, P.; El Khakani, M. A.; Chaker, M.; Amassian, A.; Martinu, L. Correlation between the sp2-phase nanostructure and the physical properties of unhydrogenated carbon nitride. J. Appl. Phys. 2005, 98, 044310.
Zhang, J. S.; Zhang, G. G.; Chen, X. F.; Lin, S.; Möhlmann, L.; Dolega, G., Lipner, G.; Antonietti, M.; Blechert, S.; Wang, X. C. Co-monomer control of carbon nitride semiconductors to optimize hydrogen evolution with visible light. Angew. Chem., Int. Ed. 2012, 51, 3183–3187.
Zhao, J.; Lin, B. N.; Zhu, Y. F.; Zhou, Y. H.; Liu, H. Y. Phosphor-doped hexagonal boron nitride nanosheets as effective acid-base bifunctional catalysts for one-pot deacetalization-Knoevenagel cascade reactions. Catal. Sci. Technol. 2018, 8, 5900–5905.
Zhang, S.; Zhao, Y. X.; Shi, R.; Waterhouse, G. I. N.; Zhang, T. R. Photocatalytic ammonia synthesis: Recent progress and future. EnergyChem 2019, 1, 100013.
Nakata, K.; Ozaki, T.; Terashima, C.; Fujishima, A.; Einaga, Y. High-yield electrochemical production of formaldehyde from CO2 and seawater. Angew. Chem., Int. Ed. 2014, 53, 871–874.
Stephan, D. W.; Erker, G. Frustrated Lewis pairs: Metal-free hydrogen activation and more. Angew. Chem., Int. Ed. 2010, 49, 46–76.
Stephan, D. W.; Erker, G. Frustrated Lewis pair chemistry of carbon, nitrogen and sulfur oxides. Chem. Sci. 2014, 5, 2625–2641.
Mömming, C. M.; Otten, E.; Kehr, G.; Fröhlich, R.; Grimme, S.; Stephan, D. W.; Erker, G. Reversible metal-free carbon dioxide binding by frustrated Lewis pairs. Angew. Chem., Int. Ed. 2009, 48, 6643–6646.
Woinska1, M.; Milowska, K. Z.; Majewski, J. A. Electronic structure of graphene functionalized with boron and nitrogen. Phys. Status Solidi (C) 2013, 10, 1167–1171.
Shi, P. H.; Su, R. J.; Wan, F. Z.; Zhu, M. C.; Li, D. X.; Xu, S. H. Co3O4 nanocrystals on graphene oxide as a synergistic catalyst for degradation of Orange II in water by advanced oxidation technology based on sulfate radicals. Appl. Catal. B: Environ. 2012, 123–124, 265–272.
Jeong, H. M.; Lee, J. W.; Shin, W. H.; Choi, Y. J.; Shin, H. J.; Kang, J. K.; Choi, J. W. Nitrogen-doped graphene for highperformance ultracapacitors and the importance of nitrogen-doped sites at basal planes. Nano Lett. 2011, 11, 2472–2477.
Wang, Y.; Shao, Y. Y.; Matson, D. W.; Li, J. H.; Lin, Y. H. Nitrogen-doped graphene and its application in electrochemical biosensing. ACS Nano 2010, 4, 1790–1798.
Bepete, G.; Voiry, D.; Chhowalla, M.; Chiguvare, Z.; Coville, N. J. Incorporation of small BN domains in graphene during CVD using methane, boric acid and nitrogen gas. Nanoscale 2013, 5, 6552–6557.
Chen, X.; Qiao, Q. A.; An, L.; Xia, D. G. Why do boron and nitrogen doped a-and γ-graphyne exhibit different oxygen reduction mechanism? A first-principles study. J. Phys. Chem. C 2015, 119, 11493–11498.
Q.; Su, J. C.; Chen, H. L.; Wang, D. Q.; Tian, X. Y.; Zhang, Y. J.; Feng, X.; Wang, S.; Li, J.; Jin, H. L. Highly conductive nitrogen-doped sp2/sp3 hybrid carbon as a conductor-free charge storage host. Adv. Funct. Mater. 2022, 32, 2209201.
Steiner, U. B.; Caseri, W. R.; Suter, U. W.; Rehahn, M.; Schmitz, L. Ultrathin layers of low-and high-molecular-weight imides on gold and copper. Langmuir 1993, 9, 3245–3254.
Zeng, L.; Dai, C. H.; Liu, B.; Xue, C. Oxygen-assisted stabilization of single-atom Au during photocatalytic hydrogen evolution. J. Mater. Chem. A 2019, 7, 24217–24221.
Duan, X. P.; Tian, X. L.; Ke, J. H.; Yin, Y.; Zheng, J. W.; Chen, J.; Cao, Z. M.; Xie, Z. X.; Yuan, Y. Z. Size controllable redispersion of sintered Au nanoparticles by using iodohydrocarbon and its implications. Chem. Sci. 2016, 7, 3181–3187.
Kim, D.; Shin, D.; Heo, J.; Lim, H.; Lim, J. A.; Jeong, H. M.; Kim, B. S.; Heo, I.; Oh, I.; Lee, B. et al. Unveiling electrode-electrolyte design-based NO reduction for NH3 synthesis. ACS Energy Lett. 2020, 5, 3647–3656.
Zhou, Q.; Gong, F.; Xie, Y. L.; Xia, D. W.; Hu, Z. G.; Wang, S. J.; Liu, L. S.; Xiao, R. A general strategy for designing metal-free catalysts for highly-efficient nitric oxide reduction to ammonia. Fuel 2022, 310, 122442.
Luo, Y. J.; Chen, K.; Shen, P.; Li, X. C.; Li, X. T.; Li, Y. H.; Chu, K. B-doped MoS2 for nitrate electroreduction to ammonia. J. Colloid Interf. Sci. 2023, 629, 950–957.
Yang, C. H.; Zhu, Y. T.; Liu, J. Q.; Qin, Y. C.; Wang, H. Q.; Liu, H. L.; Chen, Y. N.; Zhang, Z. C.; Hu, W. P. Defect engineering for electrochemical nitrogen reduction reaction to ammonia. Nano Energy 2020, 77, 105126.
Ouyang, W. C.; Zhi, Q. M.; Gong, L. L.; Sun, H.; Liu, M. H.; Zhang, J.; Han, X.; Xia, Z. H.; Zhang, L. P. Rational design of boron-containing co-doped graphene as highly efficient electro-catalysts for the nitrogen reduction reaction. J. Mater. Chem. A 2021, 9, 24590–24599.
Jiao, J. Q.; Wei, Y. C.; Zhao, Z.; Zhong, W. J.; Liu, J.; Li, J. M.; Duan, A. J.; Jiang, G. Y. Synthesis of 3D ordered macroporous TiO2-supported Au nanoparticle photocatalysts and their photocatalytic performances for the reduction of CO2 to methane. Catal. Today 2015, 258, 319–326.
Margitfalvi, J. L.; Fási, A.; Hegedűs, M.; Lónyi, F.; Gőbölös, S.; Bogdanchikova, N. Au/MgO catalysts modified with ascorbic acid for low temperature CO oxidation. Catal. Today 2002, 72, 157–169.
Veith, G. M.; Lupini, A. R.; Pennycook, S. J.; Ownby, G. W.; Dudney, N. J. Nanoparticles of gold on γ-Al2O3 produced by dc magnetron sputtering. J. Catal. 2005, 231, 151–158.
Zhao, L. Y.; Levendorf, M.; Goncher, S.; Schiros, T.; Pálová, L.; Zabet-Khosousi, A.; Rim, K. T.; Gutiérrez, C.; Nordlund, D.; Jaye, C. et al. Local atomic and electronic structure of boron chemical doping in monolayer graphene. Nano Lett. 2013, 13, 4659–4665.
Ferrighi, L.; Trioni, M. I.; Di Valentin, C. Boron-doped, nitrogen-doped, and codoped graphene on Cu (111): A DFT + vdW study. J. Phys. Chem. C 2015, 119, 6056–6064.
Matkovich, V. I. Boron and Refractory Borides; Springer: Berlin, Heidelberg, 1977; pp 1–656.
D. Y.; Jeon, W.; Tu, N. D. K.; Yeo, S. Y.; Lee, S. S.; Sung, B. J.; Chang, H.; Lim, J. A.; Kim, H. High-concentration boron doping of graphene nanoplatelets by simple thermal annealing and their supercapacitive properties. Sci. Rep. 2015, 5, 9817.
Wen, G. L.; Zhao, W.; Chen, X.; Liu, J. Q.; Wang, Y.; Zhang, Y.; Huang, Z. J.; Wu, Y. C. N-doped reduced graphene oxide/MnO2 nanocomposite for electrochemical detection of Hg2+ by square wave stripping voltammetry. Electrochim. Acta 2018, 291, 95–102.
Zhao, Q.; Wu, W. X.; Wei, X. Y.; Jiang, S. L.; Zhou, T.; Li, Q.; Lu, Q. Graphitic carbon nitride as electrode sensing material for tetrabromobisphenol-A determination. Sensors Actuat. B: Chem. 2017, 248, 673–681.
Kong, Y.; Li, Y.; Yang, B.; Li, Z. J.; Yao, Y.; Lu, J. G.; Lei, L. C.; Wen, Z. H.; Shao, M. H.; Hou, Y. Boron and nitrogen co-doped porous carbon nanofibers as metal-free electrocatalysts for highly efficient ammonia electrosynthesis. J. Mater. Chem. A 2019, 7, 26272–26278.
Wan, H.; Bagger, A.; Rossmeisl, J. Electrochemical nitric oxide reduction on metal surfaces. Angew. Chem. 2021, 133, 22137–22143.
Dong, W. F.; Zhang, N.; Li, S. X.; Min, S. X.; Peng, J.; Liu, W. Y.; Zhan, D. P.; Bai, H. C. A Mn single atom catalyst with Mn-N2O2 sites integrated into carbon nanosheets for efficient electrocatalytic CO2 reduction. J. Mater. Chem. A 2022, 10, 10892–10901.
Yuan, M. L.; Zhang, H. H.; Xu, Y.; Liu, R. J.; Wang, R.; Zhao, T. K.; Zhang, J. X.; Liu, Z. J.; He, H. Y.; Yang, C. et al. Artificial frustrated Lewis pairs facilitating the electrochemical N2 and CO2 conversion to urea. Chem Catal. 2022, 2, 309–320.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 22206096 and 21936005) and China Postdoctoral Science Foundation (Nos. 2020TQ0166 and 2021M691771).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
12274_2023_5943_MOESM1_ESM.pdf
Applying heteroatom co-doped carbon nanotube for manifesting high performance in the electrochemical reduction of aqueous nitrogen oxide by gold nanoparticles
Rights and permissions
About this article
Cite this article
Chung, J., Yin, H., Wang, R. et al. Applying heteroatom co-doped carbon nanotube for manifesting high performance in the electrochemical reduction of aqueous nitrogen oxide by gold nanoparticles. Nano Res. 17, 1151–1164 (2024). https://doi.org/10.1007/s12274-023-5943-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-023-5943-0