Abstract
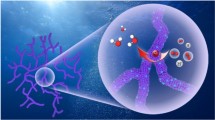
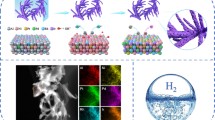
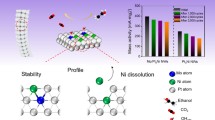
Hydrogen economy, as the most promising alternative energy system, relies on the hydrogen production through sustainable water splitting which in turn relies on the high efficiency electrocatalysts. PtAuCu A1-phase alloy has been predicted to be a promising electrocatalyst for the hydrogen evolution. As such preferred phase of Pt-Au-Cu is not thermodynamically favored, herein, we stabilize PtAuCu alloy by engineering the high-entropy phase in the form of nanowire. Density functional theory (DFT) calculations indicate that, in comparison with the ordered phase and segregated phases with discrete hydrogen binding energy, the high-entropy phase provides a diverse combination of site composition to continuously tune the hydrogen binding energy, and thus generate a series of highly active sites for the hydrogen evolution. Reflecting the theoretical prediction, electrochemical tests show that the A1-phase PtAuCu nanowire significantly outperforms its nanoparticle counterpart with phase segregation, toward the electrocatalysis of hydrogen evolution, offering one of the best hydrogen evolution electrocatalysts.

Similar content being viewed by others
References
Vesborg, P. C. K.; Seger, B.; Chorkendorff, I. Recent development in hydrogen evolution reaction catalysts and their practical implementation. J. Phys. Chem. Lett. 2015, 6, 951–957.
Li, Y. J.; Sun, Y. J.; Qin, Y. N.; Zhang, W. Y.; Wang, L.; Luo, M. C.; Yang, H.; Guo, S. J. Recent advances on water-splitting electrocatalysis mediated by noble-metal-based nanostructured materials. Adv. Energy Mater. 2020, 10, 1903120.
Subbaraman, R.; Tripkovic, D.; Strmcnik, D.; Chang, K. C.; Uchimura, M.; Paulikas, A. P.; Stamenkovic, V.; Markovic, N. M. Enhancing hydrogen evolution activity in water splitting by tailoring Li+-Ni(OH)2-Pt interfaces. Science 2011, 334, 1256–1260.
Yao, Y. G.; Huang, Z. N.; Xie, P. F.; Lacey, S. D.; Jacob, R. J.; Xie, H.; Chen, F. J.; Nie, A. M.; Pu, T. C.; Rehwoldt, M. et al. Carbothermal shock synthesis of high-entropy-alloy nanoparticles. Science 2018, 359, 1489–1494.
Yang, T. T.; Zhu, H.; Wan, M.; Dong, L.; Zhang, M.; Du, M. L. Highly efficient and durable PtCo alloy nanoparticles encapsulated in carbon nanofibers for electrochemical hydrogen generation. Chem. Commun. 2016, 52, 990–993.
Greeley, J.; Jaramillo, T. F.; Bonde, J.; Chorkendorff, I.; Nørskov, J. K. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat. Mater. 2006, 5, 909–913.
Sheng, W. C.; Myint, M.; Chen, J. G.; Yan, Y. S. Correlating the hydrogen evolution reaction activity in alkaline electrolytes with the hydrogen binding energy on monometallic surfaces. Energy Environ. Sci. 2013, 6, 1509–1512.
Danilovic, N.; Subbaraman, R.; Strmcnik, D.; Chang, K. C.; Paulikas, A. P.; Stamenkovic, V. R.; Markovic, N. M. Enhancing the alkaline hydrogen evolution reaction activity through the bifunctionality of Ni(OH)2/metal catalysts. Angew. Chem. 2012, 124, 12663–12666.
Intikhab, S.; Snyder, J. D.; Tang, M. H. Adsorbed hydroxide does not participate in the volmer step of alkaline hydrogen electrocatalysis. ACS Catal. 2017, 7, 8314–8319.
Wang, T.; Guo, Y. R.; Zhou, Z. X.; Chang, X. H.; Zheng, J.; Li, X. G. Ni-Mo nanocatalysts on N-doped graphite nanotubes for highly efficient electrochemical hydrogen evolution in acid. ACS Nano 2016, 10, 10397–10403.
Wang, H. P.; Li, X. B.; Gao, L.; Wu, H. L.; Yang, J.; Cai, L.; Ma, T. B.; Tung, C. H.; Wu, L. Z.; Yu, G. Three-dimensional graphene networks with abundant sharp edge sites for efficient electrocatalytic hydrogen evolution. Angew. Chem., Int. Ed. 2018, 57, 192–197.
Wang, X. S.; Zhu, Y. H.; Vasileff, A.; Jiao, Y.; Chen, S. M.; Song, L.; Zheng, B.; Zheng, Y.; Qiao, S. Z. Strain effect in bimetallic electrocatalysts in the hydrogen evolution reaction. ACS Energy Lett. 2018, 3, 1198–1204.
Wang, D. L.; Xin, H. L.; Hovden, R.; Wang, H. S.; Yu, Y. C.; Muller, D. A.; DiSalvo, F. J.; Abruña, H. D. Structurally ordered intermetallic platinum-cobalt core–shell nanoparticles with enhanced activity and stability as oxygen reduction electrocatalysts. Nat. Mater. 2013, 12, 81–87.
Jiang, B. B.; Tang, Z. Y.; Liao, F.; Lin, H. P.; Lu, S. K.; Li, Y. Y.; Shao, M. W. Powerful synergy: Efficient Pt-Au-Si nanocomposites as state-of-the-art catalysts for electrochemical hydrogen evolution. J. Mater. Chem. A 2017, 5, 21903–21908.
Cao, Z. M.; Chen, Q. L.; Zhang, J. W.; Li, H. Q.; Jiang, Y. Q.; Shen, S. Y.; Fu, G.; Lu, B. A.; Xie, Z. X.; Zheng, L. S. Platinum-nickel alloy excavated nano-multipods with hexagonal close-packed structure and superior activity towards hydrogen evolution reaction. Nat. Commun. 2017, 8, 15131.
Shen, Y.; Zhou, Y. F.; Wang, D.; Wu, X.; Li, J.; Xi, J. Y. Nickel-copper alloy encapsulated in graphitic carbon shells as electrocatalysts for hydrogen evolution reaction. Adv. Energy Mater. 2018, 8, 1701759.
Wei, M.; Huang, L.; Huang, S. L.; Chen, Z. Y.; Lyu, D.; Zhang, X. R.; Wang, S. B.; Tian, Z. Q.; Shen, P. K. Highly efficient Pt-Co alloy hollow spheres with ultra-thin shells synthesized via Co-B-O complex as intermediates for hydrogen evolution reaction. J. Catal. 2020, 381, 385–394.
Huang, L. L.; Chen, P. C.; Liu, M. H.; Fu, X. B.; Gordiichuk, P.; Yu, Y. N.; Wolverton, C.; Kang, Y. J.; Mirkin, C. A. Catalyst design by scanning probe block copolymer lithography. Proc. Natl. Acad. Sci. USA 2018, 115, 3764–3769.
Chen, Y.; Lai, Z. C.; Zhang, X.; Fan, Z. X.; He, Q. Y.; Tan, C. L.; Zhang, H. Phase engineering of nanomaterials. Nat. Rev. Chem. 2020, 4, 243–256.
Cheng, H. F.; Yang, N. L.; Lu, Q. P.; Zhang, Z. C.; Zhang, H. Syntheses and properties of metal nanomaterials with novel crystal phases. Adv. Mater. 2018, 30, 1707189.
Wang, J. L.; Wei, Y.; Li, H.; Huang, X.; Zhang, H. Crystal phase control in two-dimensional materials. Sci. China Chem. 2018, 61, 1227–1242.
Chen, P. C.; Du, J. S.; Meckes, B.; Huang, L. L.; Xie, Z.; Hedrick, J. L.; Dravid, V. P.; Mirkin, C. A. Structural evolution of three-component nanoparticles in polymer nanoreactors. J. Am. Chem. Soc. 2017, 139, 9876–9884.
Ye, Y. F.; Wang, Q.; Lu, J.; Liu, C. T.; Yang, Y. High-entropy alloy: Challenges and prospects. Mater. Today 2016, 19, 349–362.
Aikens, D. A. Electrochemical methods, fundamentals and applications. J. Chem. Educ. 1983, 60, A25.
Holze, R. Book review: Electrochemical methods. fundamentals and applications (2nd edition). By Allen J. Bard and Larry R. Faulkner. Angew. Chem., Int. Ed. 2002, 41, 655–657.
Chen, C.; Kang, Y. J.; Huo, Z. Y.; Zhu, Z. W.; Huang, W. Y.; Xin, H. L.; Snyder, J. D.; Li, D. G.; Herron, J. A.; Mavrikakis, M. et al. Highly crystalline multimetallic nanoframes with three-dimensional electrocatalytic surfaces. Science 2014, 343, 1339–1343.
Lombardo, D.; Calandra, P.; Pasqua, L.; Magazù, S. Self-assembly of organic nanomaterials and biomaterials: The bottom-up approach for functional nanostructures formation and advanced applications. Materials 2020, 13, 1048.
Kang, Y. J.; Murray, C. B. Synthesis and electrocatalytic properties of cubic Mn-Pt nanocrystals (nanocubes). J. Am. Chem. Soc. 2010, 132, 7568–7569.
Kang, Y. J.; Qi, L.; Li, M.; Diaz, R. E.; Su, D.; Adzic, R. R.; Stach, E.; Li, J.; Murray, C. B. Highly active Pt3Pb and core–shell Pt3Pb-Pt electrocatalysts for formic acid oxidation. ACS Nano 2012, 6, 2818–2825.
Zhan, W. C.; Wang, J. L.; Wang, H. F.; Zhang, J. S.; Liu, X. F.; Zhang, P. F.; Chi, M. F.; Guo, Y. L.; Guo, Y.; Lu, G. Z. et al. Crystal structural effect of AuCu alloy nanoparticles on catalytic CO oxidation. J. Am. Chem. Soc. 2017, 139, 8846–8854.
Kang, Y. J.; Snyder, J.; Chi, M. F.; Li, D. G.; More, K. L.; Markovic, N. M.; Stamenkovic, V. R. Multimetallic core/interlayer/shell nanostructures as advanced electrocatalysts. Nano Lett. 2014, 14, 6361–6367.
Yang, Z. B.; Sun, J.; Lu, S.; Vitos, L. A comparative study of solid-solution strengthening in Cr-Co-Ni complex concentrated alloys: The effect of magnetism. Comput. Mater. Sci. 2021, 192, 110408.
Nørskov, J. K.; Bligaard, T.; Logadottir, A.; Kitchin, J. R.; Chen, J. G.; Pandelov, S.; Stimming, U. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 2005, 152, J23–J26.
Kresse, G.; Hafner, J. Ab initio molecular dynamics for open-shell transition metals. Phys. Rev. B 1993, 48, 13115–13118.
Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186.
Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775.
Perdew, J. P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868.
Monkhorst, H. J.; Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSF-C) (Nos. 21773023 and 21972016). The authors thank Prof. Vicky Doan-Nguyen (Ohio State Univ.) and Dr. James P. Horwath (Univ. of Pennsylvania) for the XRD simulation.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Yu, Y., Liu, G., Jiang, S. et al. Engineering the high-entropy phase of Pt-Au-Cu nanowire for electrocatalytic hydrogen evolution. Nano Res. 16, 10742–10747 (2023). https://doi.org/10.1007/s12274-023-5868-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-023-5868-7