Abstract
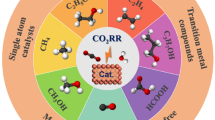
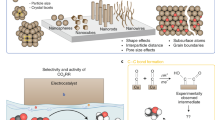
The electrocatalytic conversion of carbon dioxide (CO2) into useful fuels and chemical feedstocks is an emerging route to alleviate global warming and reduce reliance on fossil fuels. Methanol (CH3OH), as one of the most significant and widely used liquid fuels that can be generated by CO2 reduction, is essential in the chemical industry. In this minireview, we unravel the origins of the selective formation of CH3OH via CO2 reduction, including catalyst composition designs, local structure modulations, and electrolyte/catalyst interface regulations. Finally, the remaining challenges and perspectives for the CO2-to-CH3OH reduction are proposed.

Similar content being viewed by others
References
Saier, M. H. Jr. Climate change, 2007. Water Air Soil Poll. 2007, 181, 1–2.
Bushuyev, O. S.; de Luna, P.; Dinh, C. T.; Tao, L.; Saur, G.; van de Lagemaat, J.; Kelley, S. O.; Sargent, E. H. What should we make with CO2 and how can we make it. Joule 2018, 2, 825–832.
Shafaat, H. S.; Yang, J. Y. Uniting biological and chemical strategies for selective CO2 reduction. Nat. Catal. 2021, 4, 928–933.
Zhu, P.; Wang, H. T. High-purity and high-concentration liquid fuels through CO2 electroreduction. Nat. Catal. 2021, 4, 943–951.
Liu, W. C.; Baek, J.; Somorjai, G. A. The methanol economy: Methane and carbon dioxide conversion. Top. Catal. 2018, 61, 530–541.
Bozzano, G.; Manenti, F. Efficient methanol synthesis: Perspectives, technologies and optimization strategies. Prog. Energy Combust. Sci. 2016, 56, 71–105.
Tian, Z.; Wang, Y.; Zhen, X. D.; Liu, Z. B. The effect of methanol production and application in internal combustion engines on emissions in the context of carbon neutrality: A review. Fuel 2022, 320, 123902.
Kothandaraman, J.; Kar, S.; Goeppert, A.; Sen, R.; Prakash, G. K. S. Advances in homogeneous catalysis for low temperature methanol reforming in the context of the methanol economy. Top. Catal. 2018, 61, 542–559.
Olah, G. A. After oil and gas: Methanol economy. Catal. Lett. 2004, 93, 1–2.
Olah, G. A. Beyond oil and gas: The methanol economy. Angew. Chem., Int. Ed. 2005, 44, 2636–2639.
Han, L. L.; Song, S. J.; Liu, M. J.; Yao, S. Y.; Liang, Z. X.; Cheng, H.; Ren, Z. H.; Liu, W.; Lin, R. Q.; Qi, G. C. et al. Stable and efficient single-atom Zn catalyst for CO2 reduction to CH4. J. Am. Chem. Soc. 2020, 142, 12563–12567.
Wang, Y. H.; Wang, Z. Y.; Dinh, C. T.; Li, J.; Ozden, A.; Golam Kibria, M.; Seifitokaldani, A.; Tan, C. S.; Gabardo, C. M.; Luo, M. C. et al. Catalyst synthesis under CO2 electroreduction favours faceting and promotes renewable fuels electrosynthesis. Nat. Catal. 2020, 3, 98–106.
Back, S.; Jung, Y. TiC- and TiN-supported single-atom catalysts for dramatic improvements in CO2 electrochemical reduction to CH4. ACS Energy Lett. 2017, 2, 969–975.
Ge, L.; Rabiee, H.; Li, M. R.; Subramanian, S.; Zheng, Y.; Lee, J. H.; Burdyny, T.; Wang, H. Electrochemical CO2 reduction in membrane-electrode assemblies. Chem 2022, 8, 663–692.
Pan, F. P.; Yang, Y. Designing CO2 reduction electrode materials by morphology and interface engineering. Energy Environ. Sci. 2020, 13, 2275–2309.
Zhang, S. Z.; Jing, X. C.; Wang, Y. H.; Li, F. W. Towards carbonneutral methanol production from carbon dioxide electroreduction. ChemNanoMat 2021, 7, 728–736.
Liu, Y. R.; Li, F. F.; Zhang, X. P.; Ji, X. Y. Recent progress on electrochemical reduction of CO2 to methanol. Curr. Opin. Green Sustain. Chem. 2020, 23, 10–17.
Peterson, A. A.; Abild-Pedersen, F.; Studt, F.; Rossmeisl, J.; Nørskov, J. K. How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energy Environ. Sci. 2010, 3, 1311–1315.
Nie, X. W.; Esopi, M. R.; Janik, M. J.; Asthagiri, A. Selectivity of CO2 reduction on copper electrodes: The role of the kinetics of elementary steps. Angew. Chem., Int. Ed. 2013, 52, 2459–2462.
Low, Q. H.; Loo, N. W. X.; Calle-Vallejo, F.; Yeo, B. S. Enhanced electroreduction of carbon dioxide to methanol using zinc dendrites pulse-deposited on silver foam. Angew. Chem., Int. Ed. 2019, 58, 2256–2260.
Kong, S. Y.; Lv, X. M.; Wang, X.; Liu, Z. Z.; Li, Z. C.; Jia, B. Q.; Sun, D.; Yang, C.; Liu, L. J.; Guan, A. X. et al. Delocalization state-induced selective bond breaking for efficient methanol electrosynthesis from CO2. Nat. Catal. 2023, 6, 6–15.
Zhai, L. N.; Cui, C. N.; Zhao, Y. T.; Zhu, X. L.; Han, J. Y.; Wang, H.; Ge, Q. F. Titania-modified silver electrocatalyst for selective CO2 reduction to CH3OH and CH4 from DFT study. J. Phys. Chem. C 2017, 121, 16275–16282.
Kuhl, K. P.; Hatsukade, T.; Cave, E. R.; Abram, D. N.; Kibsgaard, J.; Jaramillo, T. F. Electrocatalytic conversion of carbon dioxide to methane and methanol on transition metal surfaces. J. Am. Chem. Soc. 2014, 136, 14107–14113.
Nie, X. W.; Luo, W. J.; Janik, M. J.; Asthagiri, A. Reaction mechanisms of CO2 electrochemical reduction on Cu (111) determined with density functional theory. J. Catal. 2014, 312, 108–122.
Zhang, G.; Wang, T.; Zhang, M. M.; Li, L. L.; Cheng, D. F.; Zhen, S. Y.; Wang, Y. T.; Qin, J.; Zhao, Z. J.; Gong, J. L. Selective CO2 electroreduction to methanol via enhanced oxygen bonding. Nat. Commun. 2022, 13, 7768.
Kuhl, K. P.; Cave, E. R.; Abram, D. N.; Jaramillo, T. F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 2012, 5, 7050–7059.
Hazarika, J.; Manna, M. S. Electrochemical reduction of CO2 to methanol with synthesized Cu2O nanocatalyst: Study of the selectivity. Electrochim. Acta 2019, 328, 135053.
Yang, D. X.; Zhu, Q. G.; Chen, C. J.; Liu, H. Z.; Liu, Z. M.; Zhao, Z. J.; Zhang, X. Y.; Liu, S. J.; Han, B. X. Selective electroreduction of carbon dioxide to methanol on copper selenide nanocatalysts. Nat. Commun. 2019, 10, 677.
Usman, M.; Humayun, M.; Garba, M. D.; Ullah, L.; Zeb, Z.; Helal, A.; Suliman, M. H.; Alfaifi, B. Y.; Iqbal, N.; Abdinejad, M. et al. Electrochemical reduction of CO2: A review of cobalt based catalysts for carbon dioxide conversion to fuels. Nanomaterials 2021, 11, 2029.
Huang, J. Z.; Guo, X. R.; Yue, G. Q.; Hu, Q.; Wang, L. S. Boosting CH3OH production in electrocatalytic CO2 reduction over partially oxidized 5 nm cobalt nanoparticles dispersed on single-layer nitrogen-doped graphene. ACS Appl. Mater. Interfaces 2018, 10, 44403–44414.
Ulissi, Z. W.; Tang, M. T.; Xiao, J. P.; Liu, X. Y.; Torelli, D. A.; Karamad, M.; Cummins, K.; Hahn, C.; Lewis, N. S.; Jaramillo, T. F. et al. Machine-learning methods enable exhaustive searches for active bimetallic facets and reveal active site motifs for CO2 reduction. ACS Catal. 2017, 7, 6600–6608.
Zhu, W. L.; Tackett, B. M.; Chen, J. G.; Jiao, F. Bimetallic electrocatalysts for CO2 reduction. Top. Curr. Chem. 2018, 376, 41.
Lu, L.; Sun, X. F.; Ma, J.; Yang, D. X.; Wu, H. H.; Zhang, B. X.; Zhang, J. L.; Han, B. X. Highly efficient electroreduction of CO2 to methanol on palladium-copper bimetallic aerogels. Angew. Chem., Int. Ed. 2018, 57, 14149–14153.
Sun, X. F.; Zhu, Q. G.; Kang, X. C.; Liu, H. Z.; Qian, Q. L.; Zhang, Z. F.; Han, B. X. Molybdenum-bismuth bimetallic chalcogenide nanosheets for highly efficient electrocatalytic reduction of carbon dioxide to methanol. Angew. Chem., Int. Ed. 2016, 55, 6771–6775.
Guo, W. W.; Liu, S. J.; Tan, X. X.; Wu, R. Z.; Yan, X. P.; Chen, C. J.; Zhu, Q. G.; Zheng, L. R.; Ma, J. Y.; Zhang, J. et al. Highly efficient CO2 electroreduction to methanol through atomically dispersed Sn coupled with defective CuO catalysts. Angew. Chem., Int. Ed. 2021, 60, 21979–21987.
Huang, W. J.; Yuan, G. A composite heterogeneous catalyst C-Py-Sn-Zn for selective electrochemical reduction of CO2 to methanol. Electrochem. Commun. 2020, 118, 106789.
Wang, L. W.; Xu, Y. D.; Chen, T.; Wei, D. L.; Guo, X. F.; Peng, L. M.; Xue, N. H.; Zhu, Y.; Ding, M. N.; Ding, W. P. Ternary heterostructural CoO/CN/Ni catalyst for promoted CO2 electroreduction to methanol. J. Catal. 2021, 393, 83–91.
Li, P. S.; Bi, J. H.; Liu, J. Y.; Zhu, Q. G.; Chen, C. J.; Sun, X. F.; Zhang, J. L.; Han, B. X. In situ dual doping for constructing efficient CO2-to-methanol electrocatalysts. Nat. Commun. 2022, 13, 1965.
Wu, J. J.; Ma, S. C.; Sun, J.; Gold, J. I.; Tiwary, C.; Kim, B.; Zhu, L. Y.; Chopra, N.; Odeh, I. N.; Vajtai, R. et al. A metal-free electrocatalyst for carbon dioxide reduction to multi-carbon hydrocarbons and oxygenates. Nat. Commun. 2016, 7, 13869.
Liu, Y. M.; Zhang, Y. J.; Cheng, K.; Quan, X.; Fan, X. F.; Su, Y.; Chen, S.; Zhao, H. M.; Zhang, Y. B.; Yu, H. T. Selective electrochemical reduction of carbon dioxide to ethanol on a boron- and nitrogen-co-doped nanodiamond. Angew. Chem., Int. Ed. 2017, 56, 15607–15611.
Liu, Y. M.; Chen, S.; Quan, X.; Yu, H. T. Efficient electrochemical reduction of carbon dioxide to acetate on nitrogen-doped nanodiamond. J. Am. Chem. Soc. 2015, 137, 11631–11636.
Mou, S. Y.; Wu, T. W.; Xie, J. F.; Zhang, Y. Ji, L.; Huang, H.; Wang, T.; Luo, Y. L.; Xiong, X. L.; Tang, B.; Sun, X. P. Boron phosphide nanoparticles: A nonmetal catalyst for high-selectivity electrochemical reduction of CO2 to CH3OH. Adv. Mater. 2019, 31, 1903499.
Ross, M. B.; de Luna, P.; Li, Y. F.; Dinh, C. T.; Kim, D.; Yang, P. D.; Sargent, E. H. Designing materials for electrochemical carbon dioxide recycling. Nat. Catal. 2019, 2, 648–658.
Seh, Z. W.; Kibsgaard, J.; Dickens, C. F.; Chorkendorff, I.; Nørskov, J. K.; Jaramillo, T. F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998.
Periasamy, A. P.; Ravindranath, R.; Senthil Kumar, S. M.; Wu, W. P.; Jian, T. R.; Chang, H. T. Facet- and structure-dependent catalytic activity of cuprous oxide/polypyrrole particles towards the efficient reduction of carbon dioxide to methanol. Nanoscale 2018, 10, 11869–11880.
Yang, H. P.; Wu, Y.; Li, G. D.; Lin, Q.; Hu, Q.; Zhang, Q. L.; Liu, J. H.; He, C. X. Scalable production of efficient single-atom copper decorated carbon membranes for CO2 electroreduction to methanol. J. Am. Chem. Soc. 2019, 141, 12717–12723.
Daiyan, R.; Saputera, W. H.; Zhang, Q. R.; Lovell, E.; Lim, S.; Ng, Y. H.; Lu, X. Y.; Amal, R. 3D heterostructured copper electrode for conversion of carbon dioxide to alcohols at low overpotentials. Adv. Sustainable Syst. 2019, 3, 1800064.
Payra, S.; Shenoy, S.; Chakraborty, C.; Tarafder, K.; Roy, S. Structure-sensitive electrocatalytic reduction of CO2 to methanol over carbon-supported intermetallic PtZn nano-alloys. ACS Appl. Mater. Interfaces 2020, 12, 19402–19414.
Roy, A.; Jadhav, H. S.; Gil Seo, J. Cu2O/CuO electrocatalyst for electrochemical reduction of carbon dioxide to methanol. Electroanalysis 2021, 33, 705–712.
Malik, M. I.; Malaibari, Z. O.; Atieh, M.; Abussaud, B. Electrochemical reduction of CO2 to methanol over MWCNTs impregnated with Cu2O. Chem. Eng. Sci. 2016, 152, 468–477.
Bagchi, D.; Raj, J.; Singh, A. K.; Cherevotan, A.; Roy, S.; Manoj, K. S.; Vinod, C. P.; Peter, S. C. Structure-tailored surface oxide on Cu-Ga intermetallics enhances CO2 reduction selectivity to methanol at ultralow potential. Adv. Mater. 2022, 34, 2109426.
Marepally, B. C.; Ampelli, C.; Genovese, C.; Sayah, R.; Veyre, L.; Dalverny, C.; Thieuleux, C.; Quadrelli, E. A.; Perathoner, S.; Centi, G. Supported metallic nanoparticles prepared by an organometallic route to boost the electrocatalytic conversion of CO2. J. CO2Util. 2021, 50, 101613.
Han, N.; Wang, Y.; Ma, L.; Wen, J. G.; Li, J.; Zheng, H. C.; Nie, K. Q.; Wang, X. X.; Zhao, F. P.; Li, Y. F. et al. Supported cobalt polyphthalocyanine for high-performance electrocatalytic CO2 reduction. Chem 2017, 3, 652–664.
Kramer, W. W.; McCrory, C. C. L. Polymer coordination promotes selective CO2 reduction by cobalt phthalocyanine. Chem. Sci. 2016, 7, 2506–2515.
Wu, Y. S.; Jiang, Z.; Lu, X.; Liang, Y. Y.; Wang, H. L. Domino electroreduction of CO2 to methanol on a molecular catalyst. Nature 2019, 575, 639–642.
Casado-Coterillo, C.; Marcos-Madrazo, A.; Garea, A.; Irabien, Á. An analysis of research on membrane-coated electrodes in the 2001–2019 period: Potential application to CO2 capture and utilization. Catalysts 2020, 10, 1226.
Marcos-Madrazo, A.; Casado-Coterillo, C.; Irabien, Á. Sustainable membrane-coated electrodes for CO2 electroreduction to methanol in alkaline media. ChemElectroChem 2019, 6, 5273–5282.
Tang, T. M.; Wang, Z. L.; Guan, J. Q. Optimizing the electrocatalytic selectivity of carbon dioxide reduction reaction by regulating the electronic structure of single-atom M-N-C materials. Adv. Funct. Mater. 2022, 32, 2111504.
Han, S. G.; Ma, D. D.; Zhu, Q. L. Atomically structural regulations of carbon-based single-atom catalysts for electrochemical CO2 reduction. Small Methods 2021, 5, 2100102.
Wang, J. J.; Wang, G. J.; Zhang, J. F.; Wang, Y. D.; Wu, H.; Zheng, X. R.; Ding, J.; Han, X. P.; Deng, Y. D.; Hu, W. B. Inversely tuning the CO2 electroreduction and hydrogen evolution activity on metal oxide via heteroatom doping. Angew. Chem., Int. Ed. 2021, 60, 7602–7606.
Deng, B. W.; Huang, M.; Zhao, X. L.; Mou, S. Y.; Dong, F. Interfacial electrolyte effects on electrocatalytic CO2 reduction. ACS Catal. 2022, 12, 331–362.
Arán-Ais, R. M.; Gao, D. F.; Roldan Cuenya, B. Structure- and electrolyte-sensitivity in CO2 electroreduction. Acc. Chem. Res. 2018, 51, 2906–2917.
Marcandalli, G.; Monteiro, M. C. O.; Goyal, A.; Koper, M. T. M. Electrolyte effects on CO2 electrochemical reduction to CO. Acc. Chem. Res. 2022, 55, 1900–1911.
Xu, A.; Govindarajan, N.; Kastlunger, G.; Vijay, S.; Chan, K. R. Theories for electrolyte effects in CO2 electroreduction. Acc. Chem. Res. 2022, 55, 495–503.
Kim, Y. G.; Baricuatro, J. H.; Soriaga, M. P. Surface reconstruction of polycrystalline Cu electrodes in aqueous KHCO3 electrolyte at potentials in the early stages of CO2 reduction. Electrocatalysis 2018, 9, 526–530.
Kim, Y. G.; Baricuatro, J. H.; Javier, A.; Gregoire, J. M.; Soriaga, M. P. The evolution of the polycrystalline copper surface, first to Cu (111) and then to Cu (100), at a fixed CO2RR potential: A study by operando EC-STM. Langmuir 2014, 30, 15053–15056.
Zhao, S. F.; Horne, M.; Bond, A. M.; Zhang, J. Is the imidazolium cation a unique promoter for electrocatalytic reduction of carbon dioxide. J. Phys. Chem. C 2016, 120, 23989–24001.
Kumeda, T.; Tajiri, H.; Sakata, O.; Hoshi, N.; Nakamura, M. Effect of hydrophobic cations on the oxygen reduction reaction on single-crystal platinum electrodes. Nat. Commun. 2018, 9, 4378.
Banerjee, S.; Zhang, Z. Q.; Hall, A. S.; Thoi, V. S. Surfactant perturbation of cation interactions at the electrode–electrolyte interface in carbon dioxide reduction. ACS Catal. 2020, 10, 9907–9914.
Albo, J.; Beobide, G.; Castaño, P.; Irabien, A. Methanol electrosynthesis from CO2 at Cu2O/ZnO prompted by pyridine-based aqueous solutions. J. CO2Util. 2017, 18, 164–172.
Yan, Y.; Zeitler, E. L.; Gu, J.; Hu, Y.; Bocarsly, A. B. Electrochemistry of aqueous pyridinium: Exploration of a key aspect of electrocatalytic reduction of CO2 to methanol. J. Am. Chem. Soc. 2013, 135, 14020–14023.
Lee, J. H. Q.; Lauw, L. S. J.; Webster, R. D. The electrochemical reduction of carbon dioxide (CO2) to methanol in the presence of pyridoxine (vitamin B6). Electrochem. Commun. 2016, 64, 69–73.
Rabiee, A.; Nematollahi, D. Pyridinium-facilitated CO2 electroreduction on Pt nanowire: Enhanced electrochemical performance in CO2 conversion. Environ. Prog. Sustainable 2019, 38, 112–117.
MacFarlane, D. R.; Forsyth, M.; Howlett, P. C.; Kar, M.; Passerini, S.; Pringle, J. M.; Ohno, H.; Watanabe, M.; Yan, F.; Zheng, W. J. et al. Ionic liquids and their solid-state analogues as materials for energy generation and storage. Nat. Rev. Mater. 2016, 1, 15005.
Singh, S. K.; Savoy, A. W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038.
Cui, Y. D.; He, B.; Liu, X. M.; Sun, J. Ionic liquids-promoted electrocatalytic reduction of carbon dioxide. Ind. Eng. Chem. Res. 2020, 59, 20235–20252.
Jutz, F.; Andanson, J. M.; Baiker, A. Ionic liquids and dense carbon dioxide: A beneficial biphasic system for catalysis. Chem. Rev. 2011, 111, 322–353.
Zhu, Q. G.; Ma, J.; Kang, X. C.; Sun, X. F.; Liu, H. Z.; Hu, J. Y.; Liu, Z. M.; Han, B. X. Efficient reduction of CO2 into formic acid on a lead or tin electrode using an ionic liquid catholyte mixture. Angew. Chem., Int. Ed. 2016, 55, 9012–9016.
Koper, M. T. M. Theory of multiple proton–electron transfer reactions and its implications for electrocatalysis. Chem. Sci. 2013, 4, 2710–2723.
Sa, Y. J.; Lee, C. W.; Lee, S. Y.; Na, J.; Lee, U.; Hwang, Y. J. Catalyst–electrolyte interface chemistry for electrochemical CO2 reduction. Chem. Soc. Rev. 2020, 49, 6632–6665.
Jiwanti, P. K.; Natsui, K.; Nakata, K.; Einaga, Y. Selective production of methanol by the electrochemical reduction of CO2 on boron-doped diamond electrodes in aqueous ammonia solution. RSC Adv. 2016, 6, 102214–102217.
Liu, X. Y.; Li, B. Q.; Ni, B.; Wang, L.; Peng, H. J. A perspective on the electrocatalytic conversion of carbon dioxide to methanol with metallomacrocyclic catalysts. J. Energy Chem. 2022, 64, 263–275.
Fan, L.; Xia, C.; Yang, F. Q.; Wang, J.; Wang, H. T.; Lu, Y. Y. Strategies in catalysts and electrolyzer design for electrochemical CO2 reduction toward C2+ products. Sci. Adv. 2020, 6, eaay3111.
Yoshio, H.; Katsuhei, K.; Shin, S. Production of CO and CH4 in electrochemical reduction of CO2 at metal electrodes in aqueous hydrogencarbonate solution. Chem. Lett. 1985, 14, 1695–1698.
Zhang, L. X.; Hu, S. Q.; Zhu, X. F.; Yang, W. S. Electrochemical reduction of CO2 in solid oxide electrolysis cells. J. Energy Chem. 2017, 26, 593–601.
Zhao, C. C.; Wang, J. L. Electrochemical reduction of CO2 to formate in aqueous solution using electro-deposited Sn catalysts. Chem. Eng. J. 2016, 293, 161–170.
Liu, K.; Smith, W. A.; Burdyny, T. Introductory guide to assembling and operating gas diffusion electrodes for electrochemical CO2 reduction. ACS Energy Lett. 2019, 4, 639–643.
Weekes, D. M.; Salvatore, D. A.; Reyes, A.; Huang, A.; Berlinguette, C. P. Electrolytic CO2 reduction in a flow cell. Acc. Chem. Res. 2018, 51, 910–918.
Birdja, Y. Y.; Pérez-Gallent, E.; Figueiredo, M. C.; Göttle, A. J.; Calle-Vallejo, F.; Koper, M. T. M. Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels. Nat. Energy 2019, 4, 732–745.
Zhang, Z.; Huang, X.; Chen, Z.; Zhu, J. J.; Endrődi, B.; Janáky, C.; Deng, D. H. Membrane electrode assembly for electrocatalytic CO2 reduction: Principle and application. Angew. Chem., Int. Ed. 2023, e202302789.
Tackett, B. M.; Gomez, E.; Chen, J. G. Net reduction of CO2 via its thermocatalytic and electrocatalytic transformation reactions in standard and hybrid processes. Nat. Catal. 2019, 2, 381–386.
Ponsard, L.; Nicolas, E.; Tran, N. H.; Lamaison, S.; Wakerley, D.; Cantat, T.; Fontecave, M. Coupling electrocatalytic CO2 reduction with thermocatalysis enables the formation of a lactone monomer. ChemSusChem 2021, 14, 2198–2204.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 22178104, U22B20143, 21838003, and 22008069), Shanghai Municipal Science and Technology Major Project, the Shanghai Scientific and Technological Innovation Project (No. 22dz1205900), “the Fundamental Research Funds for the Central Universities”, Shanghai Rising-Star Program (No. 23QA1402200), and the Shanghai Sailing Program (No. 20YF1410200).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, T., Wang, Y., Li, Y. et al. The origins of catalytic selectivity for the electrochemical conversion of carbon dioxide to methanol. Nano Res. 17, 5–17 (2024). https://doi.org/10.1007/s12274-023-5653-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-023-5653-7