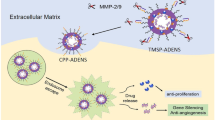
Abstract
Traditional surgical treatment is difficult to thoroughly remove esophageal squamous cell carcinomas (ESCC), and postoperative recurrence caused by residual tumor cells is a critical factor in the poor prognosis. Since surgical resection promotes the local angiogenesis at the tumor site, further exacerbating the proliferation and invasion of residual tumor cells, it is urgent to inhibit angiogenesis after surgery. Here, a functional peptide-based nanomedicine was obtained from peptide—drug conjugates, which are composed of a hydrophilic targeting motif (vascular endothelial growth factor family and their receptors (VEGFR) targeting peptide for anti-angiogenesis), and an ester-linked hydrophobic oridonin (ORI). The nanomedicine exhibits esterase-catalyzed disassembly and drug release, and significantly enhanced the anti-tumor efficacy of chemotherapeutics in a postoperative tumor recurrence model through synergistic anti-angiogenic strategies. This study provides an integrated solution for anti-angiogenesis-augmented chemotherapy and demonstrates the encouraging potential for postoperative treatment.

Similar content being viewed by others
Change history
13 April 2023
An Erratum to this paper has been published: https://doi.org/10.1007/s12274-023-5599-9
References
Kamangar, F.; Dores, G. M.; Anderson, W. F. Patterns of cancer incidence, mortality, and prevalence across five continents: Defining priorities to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol. 2006, 24, 2137–2150.
Ohashi, S.; Miyamoto, S.; Kikuchi, O.; Goto, T.; Amanuma, Y.; Muto, M. Recent advances from basic and clinical studies of esophageal squamous cell carcinoma. Gastroenterology 2015, 149, 1700–1715.
Abnet, C. C.; Arnold, M.; Wei, W. Q. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology 2018, 154, 360–373.
Poon, R. T. P.; Fan, S. T.; Wong, J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann. Surg. 2000, 232, 10–24.
Sasaki, K.; Matsuda, M.; Ohkura, Y.; Kawamura, Y.; Hashimoto, M.; Ikeda, K.; Kumada, H.; Watanabe, G. Minimum resection margin should be based on tumor size in hepatectomy for hepatocellular carcinoma in hepatoviral infection patients. Hepatol. Res. 2013, 43, 1295–1303.
Zhang, Z. Y.; Kuang, G. Z.; Zong, S.; Liu, S.; Xiao, H. H.; Chen, X. S.; Zhou, D. F.; Huang, Y. B. Sandwich-like fibers/sponge composite combining chemotherapy and hemostasis for efficient postoperative prevention of tumor recurrence and metastasis. Adv. Mater. 2018, 30, 1803217.
Ceelen, W.; Pattyn, P.; Mareel, M. Surgery, wound healing, and metastasis: Recent insights and clinical implications. Crit. Rev. Oncol. Hematol. 2014, 89, 16–26.
Goldstein, M. R.; Mascitelli, L. Surgery and cancer promotion: Are we trading beauty for cancer? QJM An Int. J. Med. 2011, 104, 811–815.
Maniwa, Y.; Okada, M.; Ishii, N.; Kiyooka, K. Vascular endothelial growth factor increased by pulmonary surgery accelerates the growth of micrometastases in metastatic lung cancer. Chest 1998, 114, 1668–1675.
Heidemann, J.; Binion, D. G.; Domschke, W.; Kucharzik, T. Antiangiogenic therapy in human gastrointestinal malignancies. Gut 2006, 55, 1497–1511.
Jayson, G. C.; Kerbel, R.; Ellis, L. M.; Harris, A. L. Antiangiogenic therapy in oncology: Current status and future directions. Lancet 2016, 388, 518–529.
Jain, R. K. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science 2005, 307, 58–62.
Ferrara, N.; Kerbel, R. S. Angiogenesis as a therapeutic target. Nature 2005, 438, 967–974.
Kerbel, R. S. Tumor angiogenesis. N. Engl. J. Med. 2008, 358, 2039–2049.
Bailey, C. E.; Parikh, A. A. Assessment of the risk of antiangiogenic agents before and after surgery. Cancer Treat. Rev. 2018, 68, 38–46.
Ferrara, N.; Gerber, H. P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676.
Olsson, A. K.; Dimberg, A.; Kreuger, J.; Claesson-Welsh, L. VEGF receptor signalling - in control of vascular function. Nat. Rev. Mol. Cell Biol. 2006, 7, 359–371.
Min, H.; Wang, J.; Qi, Y. Q.; Zhang, Y. L.; Han, X. X.; Xu, Y.; Xu, J. C.; Li, Y.; Chen, L.; Cheng, K. M. et al. Biomimetic metal-organic framework nanoparticles for cooperative combination of antiangiogenesis and photodynamic therapy for enhanced efficacy. Adv. Mater. 2019, 31, 1808200.
Qin, S.; Li, A. P.; Yi, M.; Yu, S. N.; Zhang, M. S.; Wu, K. M. Recent advances on anti-angiogenesis receptor tyrosine kinase inhibitors in cancer therapy. J. Hematol. Oncol. 2019, 12, 27.
Sadremomtaz, A.; Mansouri, K.; Alemzadeh, G.; Safa, M.; Rastaghi, A. E.; Asghari, S. M. Dual blockade of VEGFR1 and VEGFR2 by a novel peptide abrogates VEGF-driven angiogenesis, tumor growth, and metastasis through PI3K/AKT and MAPK/ERK1/2 pathway. Biochim. Biophys. Acta Gen Subj. 2018, 1862, 2688–2700.
Fallah, A.; Sadeghinia, A.; Kahroba, H.; Samadi, A.; Heidari, H. R.; Bradaran, B.; Zeinali, S.; Molavi, O. Therapeutic targeting of angiogenesis molecular pathways in angiogenesis-dependent diseases. Biomed. Pharmacother. 2019, 110, 775–785.
Zanjanchi, P.; Asghari, S. M.; Mohabatkar, H.; Shourian, M.; Ardestani, M. S. Conjugation of VEGFR1/R2-targeting peptide with gold nanoparticles to enhance antiangiogenic and antitumoral activity. J. Nanobiotechnol. 2022, 20, 7.
Moserle, L.; Jiménez-Valerio, G.; Casanovas, O. Antiangiogenic therapies: Going beyond their limits. Cancer Discov. 2014, 4, 31–41.
Zubair, H.; Khan, M. A.; Anand, S.; Srivastava, S. K.; Singh, S.; Singh, A. P. Modulation of the tumor microenvironment by natural agents: Implications for cancer prevention and therapy. Semin. Cancer Biol. 2022, 80, 237–255.
Deng, L. J.; Qi, M.; Li, N.; Lei, Y. H.; Zhang, D. M.; Chen, J. X. Natural products and their derivatives: Promising modulators of tumor immunotherapy. J. Leukoc. Biol. 2020, 108, 493–508.
Azab, A.; Nassar, A.; Azab, A. N. Anti-inflammatory activity of natural products. Molecules 2016, 21, 1321.
Efferth, T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Semin. Cancer Biol. 2017, 46, 65–83.
Ma, N.; Zhang, Z. Y.; Liao, F. L.; Jiang, T. L.; Tu, Y. Y. The birth of artemisinin. Pharmacol. Ther. 2020, 216, 107658.
Ding, Y.; Ding, C. Y.; Ye, N.; Liu, Z. Q.; Wold, E. A.; Chen, H. Y.; Wild, C.; Shen, Q.; Zhou, J. Discovery and development of natural product oridonin-inspired anticancer agents. Eur. J. Med. Chem. 2016, 122, 102–117.
Liu, X.; Xu, J. M.; Zhou, J.; Shen, Q. Oridonin and its derivatives for cancer treatment and overcoming therapeutic resistance. Genes Dis. 2021, 8, 448–462.
Wang, Y.; Cheetham, A. G.; Angacian, G.; Su, H.; Xie, L. S.; Cui, H. G. Peptide-drug conjugates as effective prodrug strategies for targeted delivery. Adv. Drug Deliv. Rev. 2017, 110–111, 112–126.
Cooper, B. M.; Iegre, J.; O’Donovan, D.; Halvarsson, M. Ö.; Spring, D. R. Peptides as a platform for targeted therapeutics for cancer: Peptide-drug conjugates (PDCs). Chem. Soc. Rev. 2021, 50, 1480–1494.
Ji, T. J.; Ding, Y. P.; Zhao, Y.; Wang, J.; Qin, H.; Liu, X. M.; Lang, J. Y.; Zhao, R. F.; Zhang, Y. L.; Shi, J. et al. Peptide assembly integration of fibroblast-targeting and cell-penetration features for enhanced antitumor drug delivery. Adv. Mater. 2015, 27, 1865–1873.
Han, X. X.; Cheng, K. M.; Xu, Y.; Wang, Y. Z.; Min, H.; Zhang, Y. L.; Zhao, X.; Zhao, R. F.; Anderson, G. J.; Ren, L. et al. Modularly designed peptide nanoprodrug augments antitumor immunity of PD-L1 checkpoint blockade by targeting indoleamine 2, 3-dioxygenase. J. Am. Chem. Soc. 2020, 142, 2490–2496.
Zhang, H. J.; He, Q. Q.; Wang, J. J.; Wang, Y. P.; Xuan, X. Y.; Sui, M.; Zhang, Z. Z.; Hou, L. Biomimetic micelles to accurately regulate the inflammatory microenvironment for glomerulonephritis treatment. Pharmacol. Res. 2022, 181, 106263.
Zhou, G. B.; Kang, H.; Wang, L.; Gao, L.; Liu, P.; Xie, J.; Zhang, F. X.; Weng, X. Q.; Shen, Z. X.; Chen, J. et al. Oridonin, a diterpenoid extracted from medicinal herbs, targets AML1-ETO fusion protein and shows potent antitumor activity with low adverse effects on t(8;21) leukemia in vitro and in vivo. Blood 2007, 109, 3441–3450.
Kang, N.; Zhang, J. H.; Qiu, F.; Tashiro, S. I.; Onodera, S.; Ikejima, T. Inhibition of EGFR signaling augments oridonin-induced apoptosis in human laryngeal cancer cells via enhancing oxidative stress coincident with activation of both the intrinsic and extrinsic apoptotic pathways. Cancer Lett. 2010, 294, 147–158.
Jun, J. H.; Oh, J. E.; Shim, J. K.; Kwak, Y. L.; Cho, J. S. Effects of bisphenol A on the proliferation, migration, and tumor growth of colon cancer cells: In vitro and in vivo evaluation with mechanistic insights related to ERK and 5-HT3. Food Chem. Toxicol. 2021, 158, 112662.
Blanco, E.; Shen, H. F.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951.
Zhang, C.; Qu, G. W.; Sun, Y. J.; Wu, X. L.; Yao, Z.; Guo, Q. L.; Ding, Q. L.; Yuan, S. T.; Shen, Z. L.; Ping, Q. N. et al. Pharmacokinetics, biodistribution, efficacy and safety of N-octyl-O-sulfate chitosan micelles loaded with paclitaxel. Biomaterials 2008, 29, 1233–1241.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 32000998 and U2004123), the Young Elite Scientists Sponsorship Program by Henan Association for Science and Technology (No. 2022HYTP046), and the China Postdoctoral Science Foundation (Nos. 2019TQ0285, 2019M662513, 2021TQ0298, and 2022TQ0296). The authors thank Lijing Zhang and Prof. Fazhan Wang (the First Affiliated Hospital, Zhengzhou University) for assistance with the in vivo fluorescence imaging. The authors would like to acknowledge the use of resources at Center of Advanced Analysis&Gene Sequencing, Zhengzhou University and National Center for Nanoscience and Technology, and acknowledge professor Bing Jiang and Ying Zhao for helpful discussions.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2023_5396_MOESM1_ESM.pdf
Modularly designed peptide-based nanomedicine inhibits angiogenesis to enhance chemotherapy for post-surgical recurrence of esophageal squamous cell carcinomas
Rights and permissions
About this article
Cite this article
Qi, Y., Shen, J., Liu, C. et al. Modularly designed peptide-based nanomedicine inhibits angiogenesis to enhance chemotherapy for post-surgical recurrence of esophageal squamous cell carcinomas. Nano Res. 16, 7347–7354 (2023). https://doi.org/10.1007/s12274-023-5396-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-023-5396-5